Solubility and the Dissolving Process
advertisement

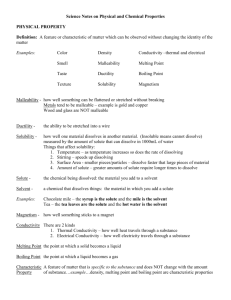
Considering the neutralization with a strong monohydroxy base, what volume of 5.00 M sulfuric acid is needed to create 2.85x1024 water molecules? Solubility and the Dissolving Process Solubility and Polarity • Solubility – the ability of one substance to dissolve into another at a given temperature and pressure; – expressed in terms of the amount of solute that will dissolve in a given amount of solvent to produce a saturated solution. • Polar compounds tend to dissolve in other polar compounds and nonpolar dissolve in other nonpolar compounds. Solubility and Polarity • “Like dissolves Like” • Miscible – describes two or more liquids that are able to dissolve into each other in various proportions. • If two substances are immiscible, than they will not mix together. Solubilities of Solids • The greater the surface area, the faster the dissolving process. • Dissolving only takes place at the surface of substances. The solvent cannot penetrate the solute, which make it take longer to dissolve bigger “chunks” • Shaking will also increase the speed. Why? Temperature • Solubilities of solids generally increase with temperature. • Increasing the temperature increases the speed of the molecules which increases the number of collisions. This speeds up the solvent’s ability to dissolve the solute. Breaking Down an Ionic Compound • The dissolving of an ionic compound involves a unique factor – The separation of ions from the lattice into individual dissolved ions. • Dissociation – the separating of a molecule into simpler molecules, atoms, radicals, or ions. • If water is the solvent then the dissociation involves hydration. • Hydration – the surrounding of the dissociated ions by water molecules. Enthalpy and Entropy • The dissociation process takes a large amount of energy to rip apart the lattice. • A large positive enthalpy change, ________, the water then surrounds it which has a large negative ΔH which nearly cancel out. • As ions are scattered and broken apart, the entropy ____________. Then the water surrounding and reorganizing the ions __________ the entropy, nearly cancelling out. Enthalpy and Entropy • The net result of all of the enthalpy and entropy changes that accompany the dissolving process determines the solubility of an ionic solid. • This study gave us our solubility rules. – Tells is substances are soluble or insoluble in water. – Other solvents may be used if needed. Saturation • When the maximum amount of solute is dissolved in solution, the solution is said to be a saturated solution. • If more can be added than is it a unsaturated solution. • Unsaturated Solution – a solution that contains less solute than a saturated solution and that is able to dissolve addition solute. Exceeding Solubility • A supersaturated solution is a solution holding more dissolved solute than what is required to reach equilibrium at a given temperature. • How can we coax the solvent to take in more solute? Equilibrium • Solubility Equilibrium – the physical state in which the opposing processes or dissolution and crystallization of a solute occur at equal rates. • The ions are leaving the solid surface at the same rate as ions are also returning to the pile of excess solute at the bottom of the solution. • Gases can also be dissolved in liquids – i.e. soda Pressure and Temperature • Henry’s Law states that the solubility of a gas increases as the partial pressure of the gas on the surface of the liquid increase. • When you open a bottle of soda the pressure is released and the solubility decreases, allowing the gases to escape and making your soda go “flat” Temperature • Warm soda has less CO2 dissolved in the liquid which also gives it a flat taste. • Gases are less soluble in a liquid of higher temperature because the increased molecular motion in the solution allows gas molecules to escape their loose arrangement with the solvent molecules. – They Break Free Concept Check • Why is ethanol miscible in water? • Why do sugar cubes dissolve more slowly in water than granulated sugar? • What factors are involved in determining the solubility of an ionic salt? • You keep adding sugar to a cold cup of coffee. You stir it by eventually you notice sugar on the bottom. Explain why no more sugar dissolves. Conductivity • Some substances have the ability to conduct an electric current. This depends on whether or not it contains charged particles that are able to move freely around the solution. • An electrolyte is a substance that dissolves in water to give the solution the ability to conduct an electric current. Conductivity • A nonelectrolyte is a liquid or solid substance that does not allow the flow of an electric current, either in solution or in its pure state, such as water or sucrose. • Ionic salts dissociate into their individual charged ions that move around the solution freely. Would this be an electrolyte or a nonelectrolyte? Acids / Bases • Strong acids COMPLETELY dissociate in water, forming the hydronium ion (H30+(aq)) – This makes all strong acids VERY good electrical conductors – Weak acids are less conductive but still dissociate to an extent. • Strong bases COMPLETELY dissociate in water, forming the hydroxide ion (_____________) – This makes strong bases also very good electrical conductors. Water and Electricity • Sea water is a great conductor. Why? • Tap water is also very good because it is not distilled (still contains ions and minerals). – Do not use electrical devices by water