Word Study Guide - Adams Science News
advertisement

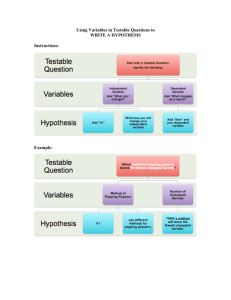
Name:____________________ Date:___________ Period:__________ Chapter 1 Practice Worksheet and Study Guide Answer each question in the worksheet below. Be sure you follow the directions for each section carefully. Multiple Choice Vocab: Circle the correct answer for the questions below. 1. Maria collected water from a spring to test for chemicals. Part of Maria’s report stated, “When I tested the solution for toxic chemicals the indicator paper did not change color”, this statement is a: a. An observation c. An inference e. None of the above b. An explanation d. A hypothesis 2. Which best describes a hypothesis? a. A proposed answer to a testable c. An observation of the living world question d. A conclusion made from data gathered b. Facts about an event in an experiment 3. What term is given to different factors that may affect the results of an experiment? a. Procedures c. Outcome b. Measurements d. Variable 4. Scientific Law: a. Well-tested explanation for a set of observations or experimental results. Explanation of patterns observed in nature. b. Describes an observed pattern in nature without attempting to explain it. c. Mass / volume d. Testable questions 5. Scientific Theory: a. Well-tested explanation for a set of observations or experimental results. Explanation of patterns observed in nature. b. Describes an observed pattern in nature without attempting to explain it. c. Mass / volume d. Testable questions 6. Manipulated Variable: a. variable that causes a change in another b. variable that does not change c. variable that changes in response to another d. Fact Testable Questions: A Testable Question can be answered by designing and conducting an experiment. 1. A good scientific question is one that can have an answer and be tested. a. For example: “Why is that a star?” is not as good as “What are stars made of?” 2. A good scientific question can be tested by some experiment or measurement that you can do. a. For example: “Where does the Sun come from? is not as good as “How will human skin react to solar radiation where one participant is covered in SPF 30 sunscreen lotion and the other participant is not covered in sunscreen lotion. 3. A good scientific question builds on what you already know. a. For example: “Will fertilizer make grass grow greener?” is not as good as “What types of fertilizer will make grass grow greener?” 4. A good scientific question, when answered, leads to other good questions. For example: “What is the flu” does not lead to as many questions as “How does the flu attack the human immune system? Determine if the following questions are testable or non-testable and write the answer in the blank provided. 1. How well do different materials sink or float in water? ____________________________________________ 2. Why do some people like sunny days instead of rainy? ___________________________________________ 3. Can changing the shape of a rocket’s fins change its flight? ________________________________________ 4. Does the sun heat freshwater and salt water at the same rate? _______________________________________ 5. Which is better Coke or Pepsi? ______________________________________________________________ 6. Do invisible unicorns exist? _________________________________________________________________ What are the 8 steps in the scientific method? Write them and explain how it works with an example study. 1. ________________________________________________________________________________________ 2. ________________________________________________________________________________________ 3. ________________________________________________________________________________________ 4. ________________________________________________________________________________________ 5. ________________________________________________________________________________________ 6. ________________________________________________________________________________________ 7. ________________________________________________________________________________________ 8. ________________________________________________________________________________________ Density: show all your work for the table below. All calculations must be labeled with the proper unit. Use calculators. Remember the formula for density is D=M/V 1. You have gathered the following data from the lab. Use the data to calculate the density of each of the liquids. Don’t forget to label your calculations with the PROPER UNITS. (SHOW ALL YOUR WORK). Samples Trial 1 Mass (g) Liquid 1 4.45 Liquid 2 3.95 5 8 10 20.16 25 Liquid 3 6.06 5 12.42 10 31.37 25 2. Volume (mL) 5 Density Trial 2 Mass (g) Volume (mL) 9.05 10 Density Graph the mass and volume data for each liquid on the graph below. Trial 3 Mass (g) Volume (mL) 22.92 25 Density 3. Draw the best-fit line for each liquid. Make sure to label each liquid and provide a key 4. What does the slope of the best-fit line represent? __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ 5. Is the slope directly or indirectly proportional? Why? __________________________________________________________________________________________________ __________________________________________________________________________________________________ __________________________________________________________________________________________________ 6. Liquids 1, 2, and 3 are insoluble in each other (they don’t mix). If you put these liquids into a graduated cylinder what would it look like? Sketch your answer below. Scientific Notation. Express the following numbers in either standard form or scientific notation form. Part A Standard Form. 1. 5.2 x 103 ______________ 5. 2.7 x 104________________ 2. 9.65 x 10-4 _____________ 6. 6.452 x 102_____________ 3. 8.5 x 10-2_______________ 7. 8.77 x 10-1 _____________ 4. 2.6 x 101________________ 8. 6.4 x 10-3 ______________ Part B Scientific notation Form 1. 78,000 ______________ 5. 16________________ 2. 0.00053 _____________ 6. 0.0043____________ 3. 250 _______________ 7. 0.875 _____________ 4. 2, 687________________ 8. 0.012654 ______________ Matching: Below match the correct term with the correct definition A. Mass are all the known digits B. Length Rise / Run C. Volume the quantity of matter in an object D. Kg SI unit for length E. Cubic meters SI unit for time F. Meters SI unit for mass G. Second SI unit for temperature H. Kelvin SI unit for volume I. formula for slope amount of space taken up by the object J. model a straight line distance between two points K. significant figures a representation of an object or event.