A.2 Heat Curves Phase diagram Worksheet Key
advertisement
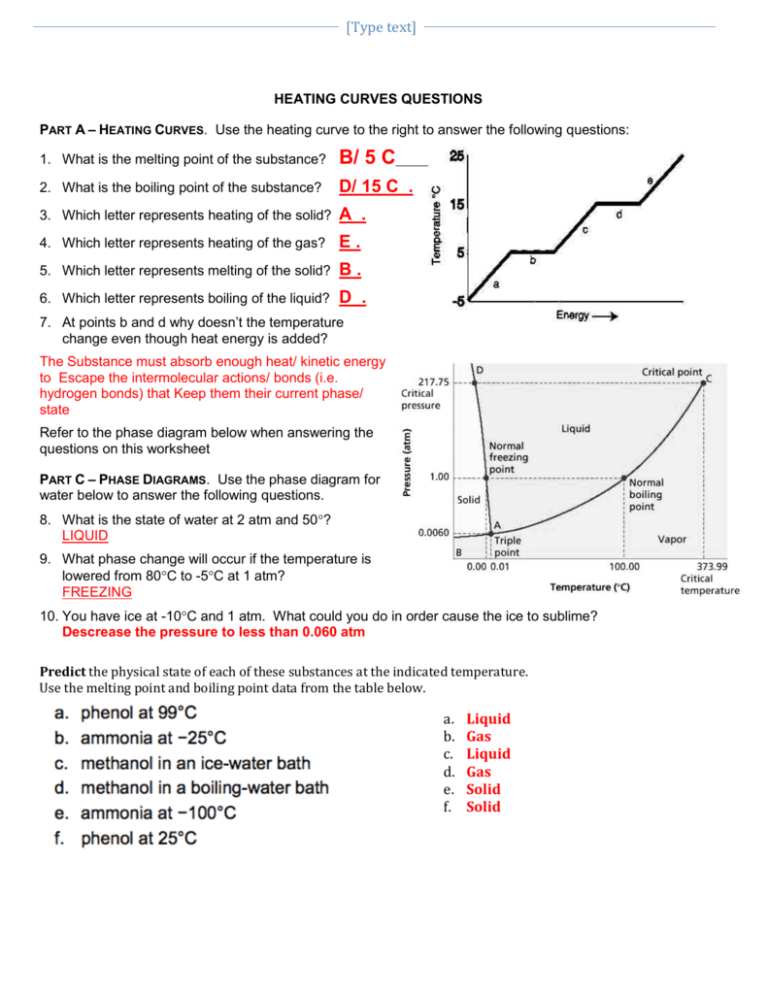
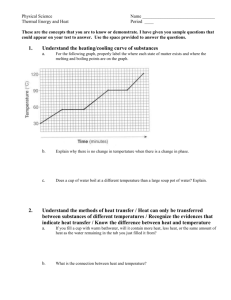
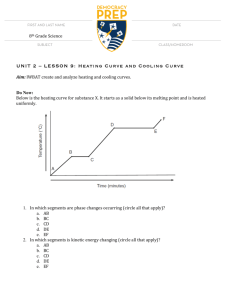
[Type text] HEATING CURVES QUESTIONS PART A – HEATING CURVES. Use the heating curve to the right to answer the following questions: 1. What is the melting point of the substance? B/ 5 C 2. What is the boiling point of the substance? D/ 15 C . 3. Which letter represents heating of the solid? A . 4. Which letter represents heating of the gas? E. 5. Which letter represents melting of the solid? B. 6. Which letter represents boiling of the liquid? D . 7. At points b and d why doesn’t the temperature change even though heat energy is added? The Substance must absorb enough heat/ kinetic energy to Escape the intermolecular actions/ bonds (i.e. hydrogen bonds) that Keep them their current phase/ state Refer to the phase diagram below when answering the questions on this worksheet PART C – PHASE DIAGRAMS. Use the phase diagram for water below to answer the following questions. 8. What is the state of water at 2 atm and 50? LIQUID 9. What phase change will occur if the temperature is lowered from 80C to -5C at 1 atm? FREEZING 10. You have ice at -10C and 1 atm. What could you do in order cause the ice to sublime? Descrease the pressure to less than 0.060 atm Predict the physical state of each of these substances at the indicated temperature. Use the melting point and boiling point data from the table below. a. b. c. d. e. f. Liquid Gas Liquid Gas Solid Solid NAME Use Table 2.1 to answer questions below. State refers to the state the substance would be in at room temperature (24 degrees Celsius) 1. Which substances in the table are in the liquid state at 125°C? Sulfur, Mercury 2. Which substances are in the gas state at 80 °C? Neon, Oxygen, Chlorine, Ethanol, Bromine 3. Which substances are a solid at 1000°C? Copper, gold Use the table below to answer the questions below. 1. Which colorless substance is a liquid at −30°C? [Type text] Ethanol 2. Which colorless substance is a gas at 60°C? Neon 3. Which substance is a solid at 7°C? Sulfur 4. As the temperature rises, which solid will melt before mercury boils? Everything 5. Use the arrangement of particles in solids and gases to explain why solids are not as easy to compress as gases. Particles are too close together and can not be pushed/ compressed closer