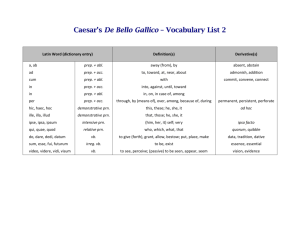
What is central

Central retinal vein occlusion
Current understanding and approaches to treatment
Prescribing information can be found on the last two slides
L.GB.01.2014.4924b
L.GB.01.2014.4924b
1
About this slide deck
This slide deck is provided as a service to medicine by Bayer
HealthCare and is intended for educational use with healthcare professionals only.
Prescribing information for Eylea
(aflibercept solution for injection) is at the end of the slide deck, and is also available in accompanying material.
Adverse events should be reported. Reporting forms and information can be found at www.yellowcard.gov.uk
Adverse events should also be reported to Bayer:
Tel: 01635 563000; E-mail: phdsguk@bayer.co.uk
2
Date of Prep March 2015
L.GB.01.2014.4924b
Acknowledgments
The contribution of the following in the development of this resource is gratefully acknowledged:
• Ben Burton, Consultant Ophthalmologist, James Paget University Hospital, Norwich
• Louise Downey, Consultant Ophthalmologist, Hull Royal Infirmary
• Nicholas Glover, Consultant Vitreoretinal Surgeon, University Hospitals, Birmingham
• Simon Kelly, Consultant Ophthalmologist Bolton NHS Trust
• Sajjad Mahmood, Consultant Ophthalmologist, Royal Eye Hospital, Manchester
• Moin Mohamed, Consultant Ophthalmological Surgeon, St Thomas’ Hospital, London
• Nishal Patel, Consultant Ophthalmologist, East Kent Hospitals University NHS Foundation Trust
• Deepali Varma, Consultant Ophthalmologist, Sunderland Eye Infirmary
•
Richard Gale, Consultant Ophthalmologist, York Teaching Hospital
• Yang Yit , Consultant Ophthalmologist, Wolverhampton Eye Hospital and Visiting Professor,
Aston University
• Sergio Pagilarini, Consultant Ophthalmologist ,University Hospitals Coventry and Warwickshire
•
Theo Empeslidis, Consultant Ophthalmologist, Leicester Royal Infirmary
• Sanjiv Banerjee, Consultant Ophthalmologist, University Hospital Wales
• Mike Williams, Consultant Ophthalmologist, Royal Victoria Infirmary, Belfast
•
Faruque Ghanchi, Consultant Ophthalmologist, Bradford Royal Infirmary
3
Date of Prep March 2015
L.GB.01.2014.4924b
Glossary
BCVA
BRVO
CFT
CRT
CRVO
Best-corrected visual acuity
Branch retinal vein occlusion
Central foveal thickness
Central retinal thickness
Central retinal vein occlusion
EDTRS
FA
IOP
LOCF
Early Treatment Diabetic Retinopathy Study
Fluorescein angiography
Intraocular pressure
Last observation carried forward
NEI VFQ-25 National Eye Institute Visual Function Questionnaire-25
OCT Optical coherence tomography
RAPD Relative afferent pupillary defect
4
Date of Prep March 2015
L.GB.01.2014.4924b
Discussion topics
• What is central retinal vein occlusion (CRVO)?
• Background and epidemiology of CRVO
• Clinical signs, symptoms and features
• Natural history and pathophysiology of CRVO
• Clinical trials of anti-VEGF therapy in CRVO
• Aflibercept development and clinical experience in CRVO
5
Date of Prep March 2015
L.GB.01.2014.4924b
What is central retinal vein occlusion?
Central retinal vein occlusion definition
• A central retinal vein occlusion (CRVO) is an occlusion of the central retinal vein in the retrolaminar region of the optic nerve head, due to thrombosis, inflammation or arteriosclerosis
Central retinal vein
Lamina cribrosa
Morley MG, Heier JS. Venous obstructive disease of the retina. In: Yanoff M, Duker JS, editors.
Ophthalmology . 3rd ed. Mosby Elsevier; 2009:597-605.
Image courtesy of Bayer HealthCare.
7
Date of Prep March 2015
L.GB.01.2014.4924b
CRVO symptoms
• Sudden acute, painless unilateral loss of vision 1
– Occasionally stepwise decline from several less severe occlusions 2
• Distorted/blurred vision 3
• Central vision decreases if macular oedema affects foveal region 4
• Affects peripheral visual fields as well as macula 5
Blurred or distorted vision typical of macular oedema following CRVO
Image courtesy of www.eylea.us.
1. Wong TY, Scott IU. N Engl J Med. 2010;363:2135-2144.
2. Hahn P, et al. Central retinal vein occlusion. In: Ryan SJ, editor. Retina.
5 th ed. Elsevier; 2013.
3. American Academy of Ophthalmology, www.geteyesmart.org/eyesmart/diseases/central-retinal-vein-occlusion-symptoms.cfm
4. Jonas JB, Lam DSC. Asia-Pac J Ophthalmol . 2012;1:355-363.
5. Hayreh, S. S.,et al Ophthalmology 2011 118 119 –133.
8
Date of Prep March 2015
L.GB.01.2014.4924b
CRVO clinical signs
• Fundoscopy may show 1,2
– Tortuous vasculature
– Scattered flame-shaped superficial retinal haemorrhages
– Retinal artery may be occluded
– ‘Blood & thunder’ appearance: widespread deep (ischaemia) and superficial haemorrhage
– Swollen disc
– Cotton wool spots (not universal)
Ischaemic CRVO
Image courtesy of
Mrs Deepali Varma, Sunderland Eye Infirmary.
• Delayed transit/slow filling on angiography 2
• Retinal thickening and in many cases submacular fluid on optical coherence tomography (OCT) 3
1. Wong TY, Scott IU. N Engl J Med. 2010;363:2135-2144.
2. Jonas JB, Lam DSC. Asia-Pac J Ophthalmol . 2012;1:355-363.
3. McAllister IL. Clin Exp Ophthalmol . 2012;40:48-58.
9
Date of Prep March 2015
L.GB.01.2014.4924b
CRVO classification
• Ischaemic CRVO 1
– Clinical presentation (BCVA* <6/60)
– Presence of relative afferent pupillary defect
– Appearance on fundoscopy 1
Multiple deep dark haemorrhages
Cotton wool spots
≥10 disc areas of non-perfusion
• Non-ischaemic (perfused) 1
• <10 disc areas of non-perfusion
• 1 in 3 non-ischaemic may progress to ischaemic over 3 years 2
• Ischaemic/non-ischaemic classification confirmed by fluorescein angiography (FA) 1
Non-ischaemic CRVO
Ischaemic CRVO
Images courtesy of Mrs Deepali Varma, Sunderland Eye Infirmary.
1. Morley MG, Heier JS. In: Ophthalmology. 3rd ed. Mosby Elsevier; 2009:597-605.
2. Central Vein Occlusion Study Group Arch Ophthalmol 1997; 115:486-491
10
Date of Prep March 2015
L.GB.01.2014.4924b
CRVO: Findings on fundoscopy
Dilated tortuous veins
Optic disk oedema
Ischaemic CRVO
• Other features
– Macular oedema
(intraretinal and subretinal fluid)
Retinal haemorrhage
Morley MG, Heier JS. In: Ophthalmology. 3rd ed. Mosby Elsevier; 2009:597-605.
Image courtesy of
Mrs Deepali Varma
Sunderland Eye Infirmary.
11
Date of Prep March 2015
L.GB.01.2014.4924b
CRVO clinical presentation
• Relative afferent pupillary defects (RAPD) differentiated ischaemic from non-ischaemic CRVO in 97% of cases 1,a
Images and animation courtesy of Bayer HealthCare.
• Normal light = both pupils are equal in size 2
• Light shines on normal eye = both pupils constrict equally 2
• Move light from normal to CRVO eye = paradoxical dilation of both eyes caused by reduced afferent input due to extent of reduced retinal perfusion 2 a When a cutoff RAPD > 0.90 log units of neutral density filters was used 3
1. Hayreh SS, et al. Ophthalmology . 2011;118:119-133.
2. Slamovits TL, et al. In: Duane’s Ophthalmology on CD-ROM . 2006.
3. Hayreh SS. Indian J Ophthalmol. 1994;42:109 –132.
12
Date of Prep March 2015
L.GB.01.2014.4924b
CRVO prevalence and incidence
Global CRVO prevalence estimated 0.80/1,000 population 1
– Standardised prevalence 0.39/1,000 in Rotterdam study 1
• Cumulative 15-year CRVO incidence 0.5% in Beaver Dam population study 2
In 1 year, 5% CRVO/BRVO (branch retinal vein occlusion) in second eye 3
Annual number new CRVO cases in UK:
14.4/100,000 population*
• 1.45x CRVO mortality risk vs.
age/gender matched controls 4
– Mainly attributable to cardiovascular disease and diabetes
1. Rogers S, et al. Ophthalmology . 2010;117:313-319.
2. Klein R, et al. Arch Ophthalmol . 2008;126:513-518.
3. McIntosh RL, et al. Ophthalmology.
2010;117:1113-1123.
*Calculated from 0.5%/15 years incidence
4. Bertelsen M, et al. Ophthalmology.
2013. Published online early.
Available at: http://www.aaojournal.org/article/S0161-6420(13)00662-3/pdf . Accessed 18 September 2013.
2
13
Date of Prep March 2015
L.GB.01.2014.4924b
Neovascular complications
• ‘100-day glaucoma’ (neovascular [NV] glaucoma
2 –3 months after primary ischaemic CRVO)
– NV glaucoma develops in 23–60% of patients with ischaemic
CRVO over 12 –15 months 1
– Severe pain (when pressure is extremely high or in acute angle closure glaucoma) 2
– Adhesions between iris and anterior chamber angle
(peripheral anterior synechiae) may cause acute angle closure glaucoma 2
• Risk of rubeosis iridis 2
1. McIntosh RL, et al. Ophthalmology.
2010;117:1113-1123.
2. Khaw PT, et al. BMJ.
2004;328:97-99.
14
Date of Prep March 2015
L.GB.01.2014.4924b
RVO risk factors
Major 1,2
• Increasing age
• Arteriosclerotic vascular risk factors:
– Hypertension
– Hyperlipidaemia
– Diabetes mellitus
– Smoking
• Glaucoma
Others 2
• Thrombophilia
• Myeloproliferative disorders
Images used with permission from Microsoft.
• Rare inflammatory conditions
1. Wong TY, Scott IU. N Engl J Med. 2010;363:2135-2144.
15
2. Royal College of Ophthalmologists Interim Guidelines for Management of Retinal Vein Occlusion. December 2010.
Date of Prep March 2015
L.GB.01.2014.4924b
Age profile of CRVO patients
80
75
72
50
40
70
60
38
47
30
20
18
16
10 7
0
Non-ischaemic CRVO (n=588) Ischaemic CRVO at 1st diagnosis (n=109)
4
24
Ischaemic CRVO (converted from non-ischaemic) (n=48)
Age range
(years)
<45
45-65
>65
Hayreh SS, et al. Ophthalmology.
2011;118:119-133.
16
Date of Prep March 2015
L.GB.01.2014.4924b
Management of risk factors
• Management of lipids, hypertension, diabetes
• Reduce risk of recurrence/occurrence of new occlusions
• Increase chance of reversing the RVO
• Ameliorate cardiovascular morbidity/mortality
• Vascular work-up
– Full blood count and ESR or plasma viscosity; urea, electrolytes, creatinine; random blood glucose; random cholesterol and HDL cholesterol; plasma protein electrophoresis; ECG; thyroid function
• Management of raised intraocular pressure
Royal College of Ophthalmologists Interim Guidelines for Management of Retinal Vein Occlusion. December 2010.
17
Date of Prep March 2015
L.GB.01.2014.4924b
Retinal vein occlusion pathogenesis
• Exact pathogenesis of
RVO is unclear
• Thrombus formation from changes to Virchow’s triad
– Haemodynamic change resulting in stasis and/or turbulence
– Vessel wall damage from injury or pathology
– Hypercoagulability
Vessel damage
Hypercoagulable state
Thrombosis
Stasis/ turbulence
Wong TY, Scott IU. N Engl J Med. 2010;363:2135-2144.
18
Date of Prep March 2015
L.GB.01.2014.4924b
CRVO pathophysiology
• Two significant complications:
– Thrombus in central retinal vein prevents venous outflow and may result in cystoid macular oedema 1
– Retinal ischaemia – associated with worse clinical outcomes 1
• Note near right angle where central retinal vein
Central retinal vein exits eye
– Haemodynamic changes 2
1. McAllister IL, et al. Clin Exp Ophthalmol. 2012;40:48-58.
2. Hahn, P., et al Central Retinal Vein Occlusion. In Retina Ed. Ryan S, Philadelphia, PA: Elsevier, 2009.
Macula
Retina
Adapted from Riordan-Eva P, Whitcher JP. Vaughan
& Asbury’s General Ophthalmology. 2008.
19
Date of Prep March 2015
L.GB.01.2014.4924b
Macular oedema pathophysiology
• Leukocytes migrate across the vascular wall and into retinal tissues 1,2
• Inflammatory mediators IL-1,
TNFα and VEGF are secreted and amplify the inflammatory response 3
IL-1
TNF-
α
VEGF
• The blood-retinal barrier breaks down, causing increased vascular permeability and fluid leakage 3
IL-1 = interleukin 1; TNF-
α = tumour necrosis factor alpha;
VEGF = vascular endothelial growth factor.
• Fluid accumulates in the retinal extracellular matrix 3
1. Hahn P et al Central Retinal Vein Occlusion in Retina 5 th edition, Ed Ryan SJ Elsevier 2013
2. Deobhakta et al Int J Inflammation 2013:, 38412.
Published online only.
3. Kent D, et al.
Br J Ophthalmol.
2000;84:542-545.
Image courtesy of DS Boyer, MD.
20
Date of Prep March 2015
L.GB.01.2014.4924b
CRVO pathophysiology
Visual loss from CRVO may occur via the following mechanisms:
• Acutely
– Retinal bleeding at the macula
– Poor perfusion causing ischaemic macula/fovea
– Macular oedema due to vascular damage, increased VEGF production and inflammation
• Chronically
– Visual loss may occur secondary to neovascularisation and vitreous haemorrhage or rubeotic glaucoma
21
Date of Prep March 2015
L.GB.01.2014.4924b
CRVO pathophysiology: retinal bleeding at the macula
Blood clot
Impaired blood flow
Increased intraluminal and interstitial pressure
Retinal haemorrhage
Acute loss of visual function
1. Karia N. Clin Ophthalmol. 2010;4:809-816.
2. Jonas JB, Lam DSC. Asia-Pac J Ophthalmol . 2012;1(6):355-363.
22
Date of Prep March 2015
L.GB.01.2014.4924b
CRVO pathophysiology: poor perfusion and ischaemia
Blood clot
Impaired blood flow
Increased intraluminal and interstitial pressure
Reduced arterial perfusion and retinal ischaemia
Hypoxia
VEGF production
Vascular permeability
Acute/chronic loss of visual function
Macular oedema
Capillary damage
1. Karia N. Clin Ophthalmol. 2010;4:809-816.
2. Jonas JB, Lam DSC. Asia-Pac J Ophthalmol . 2012;1(6):355-363.
23
Date of Prep March 2015
L.GB.01.2014.4924b
CRVO pathophysiology: macular oedema due to vascular change, VEGF expression and inflammation
Blood clot
Impaired blood flow
Increased intraluminal and interstitial pressure
Capillary damage
Ischaemia Hypoxia Inflammation
Reduced arterial perfusion and retinal ischaemia
VEGF production
Vascular permeability
Neuronal cell death
Acute/chronic loss of visual function
Macular oedema
1. Karia N. Clin Ophthalmol. 2010;4:809-816
24
Date of Prep March 2015
L.GB.01.2014.4924b
Macular oedema
• Diffuse cystoid macular oedema results from:
– Abnormal retinal capillary permeability
– Expansion of extracellular spaces
• Subretinal fluid
• Underlying aetiology is breakdown of blood-retinal barrier
Image courtesy of
Mr Simon P Kelly
Bolton, UK.
SD-OCT demonstrating cystoid macular oedema and retinal thickening.
Johnson MW. Am J Ophthalmol . 2009;147:11-21.
SD-OCT=spectral domain optical coherence tomography.
25
Date of Prep March 2015
L.GB.01.2014.4924b
Macular oedema
‘
Macular oedema, with or without macular non-perfusion, is the most frequent cause of vision loss in patients with retinal vein occlusion
’
26
Date of Prep March 2015
L.GB.01.2014.4924b
Wong TY, Scott IU. N Engl J Med. 2010;363:2135-2144.
CRVO natural history
• Occlusion of collateral vessels at the disc
• Visual loss secondary to ischaemia or macular oedema
• Baseline visual function predicts prognosis
• Chronic macular oedema may result in
– Subfoveal retinal pigment epithelial dispersion and clumping
– Photoreceptor loss
• Anterior segment neovascularisation and rubeotic glaucoma
• Loss of eye in severe cases
Non-ischaemic CRVO right posterior pole.
Multiple haemorrhages in all 4 quadrants, tortuous veins, absence of cotton wool spots suggests well-perfused non-ischaemic CRVO.
Image courtesy of Mr Simon Kelly, Bolton UK.
McAllister IL. Clin Exp Ophthalmol . 2012;40:48-58.
Ischaemic CRVO: swollen disk on colour fundoscopy
Image courtesy of Mrs Deepali Varma,
Sunderland Eye Infirmary.
Ischaemic CRVO: swollen disk on fluorescein angiography
Image courtesy of Mrs Deepali Varma,
Sunderland Eye Infirmary.
27
Date of Prep March 2015
L.GB.01.2014.4924b
CRVO natural history:
Consequences for central vision
• Visual loss in acute phase secondary to macular oedema, intraretinal macular haemorrhage, and macular ischaemia 1
• Visual acuity may improve but not beyond 20/40 2
Images courtesy of Mrs Deepali Varma, Sunderland Eye Infirmary.
Non-ischaemic CRVO with widespread haemorrhages in all 4 quadrants with engorgement of the optic disc
1. McAllister IL. Clin Exp Ophthalmol . 2012;40:48-58.
2. McIntosh RL, et al. Ophthalmology.
2010;117:1113-1123.
28
Date of Prep March 2015
L.GB.01.2014.4924b
CRVO: A high-VEGF state disease
VEGF levels in eyes with CRVO are among the highest in all retinal disorders, higher than BRVO and up to 80 times higher than wet AMD
1-6
1. Holekamp NM, et al. Am J Ophthalmol. 2002;134:220-227;
2. Duh EJ, et al. Am J Ophthalmol. 2004;137:668-674;
3. Noma H, et al. Graefes Arch Clin Exp Ophthalmol. 2010;248:1559-1565;
4. Noma H, et al. Graefes Arch Clin Exp Ophthalmol.
2006;244:309-315-
5. Asato R. Poster D977, presented at ARVO 2010
6. Noma H, et al. Eur J Ophthalmol.
2008;16:1017 -1019;
29
Date of Prep March 2015
L.GB.01.2014.4924b
Levels of vitreous VEGF in retinal disease
• VEGF levels in CRVO are up to 69x higher than in wet AMD and up to 12x higher than in BRVO
Condition
Wet AMD
Branch retinal vein occlusion
Central retinal vein occlusion
VEGF level (pg/mL)
39
–62 1,2
226-1263 3 –4
744-2692 5,6
1. Holekamp NM, et al. Am J Ophthalmol. 2002;134:220-227;
2. Duh EJ, et al. Am J Ophthalmol. 2004;137:668-674;
3. Noma H, et al. Graefes Arch Clin Exp Ophthalmol. 2010;248:1559-1565;
4. Noma H, et al. Graefes Arch Clin Exp Ophthalmol.
2006;244:309-315-
5. Asato R. Poster D977, presented at ARVO 2010
6. Noma H, et al. Eur J Ophthalmol.
2008;16:1017 -1019;
30
Date of Prep March 2015
L.GB.01.2014.4924b
CRVO studies
CRVO: Treatment strategies
Natural history 1,2
May progress to ischaemia, neovascularisation, glaucoma
Surgical 3,4
Not recommended for routine use/not evaluated in controlled clinical trials CRVO
Laser photocoagulation 5
Less efficacious, management burden
Anti-VEGF 8-12
Validated as an effective therapeutic intervention in CRVO
Steroids 6,7
Concerns with ocular adverse events
1.
Morley MG, Heier JS. In: Ophthalmology . 3rd ed. 2009:597-605;
2.
The Central Vein Occlusion Study Group. Arch Ophthalmol.
1993;111:1087-1095;
3.
Mohamed Q, et al. Ophthalmology . 2007;114:507-519;
4.
McIntosh R, et al. Ophthalmology . 2007;114:835-846;
5.
The Central Vein Occlusion Study Group. Ophthalmology.
1995;102:1425-1433;
6.
Ip MS, et al; SCORE Study Research Group. Arch Ophthalmol . 2009;127:1101-1114;
7.
Haller JA, et al. Ophthalmology . 2011;118:2453-2460;
8.
Campochiaro PA, et al. Ophthalmology . 2011;118:2041-2049;
9.
Heier JS, et al. Ophthalmology . 2012;119:802-809;
10. Brown DM, et al. Ophthalmology. 2010;117:1124-1133;
11. Holz FG, et al. Br J Ophthalmology . 2013;97:278-284;
12. Brown DM, et al. Am J Ophthalmol . 2013;155:429-437.
32
Date of Prep March 2015
L.GB.01.2014.4924b
CRVO: Surgical interventions
• The safety and efficacy of surgical treatments for CRVO have not been evaluated in randomised clinical trials 1
– Vitrectomy may increase retinal oxygenation and relieve macular traction 1
– Radial optic neurotomy (RON) may relieve pressure on the occluded vein 1
– Laser-induced chorioretinal anastomosis bypasses the occluded central retinal vein to create another outflow 2
– Haemodilution increased visual acuity vs. control in a randomised trial, but requires careful patient selection and inpatient stay 1,3
Pars plana vitrectomy.
Illustration courtesy of Bayer HealthCare.
1. Mohamed Q, et al. Ophthalmology . 2007;114(3):507-519.
2. McAllister IL, et al. Ophthalmology . 2010;117(5):954-965.
3. Glacet-Bernard A, et al. Graefe’s Arch Clin Exp Ophthalmol. 2011;294:505-12.
33
Date of Prep March 2015
L.GB.01.2014.4924b
CRVO: Milestones in treatment*
Laser
Photocoagulation
1977 1984
Steroids
1997
2004
Treatment first used
Trial data first published
Anti-VEGF
2007 2009 2010 2011 2012 2013
1. The Central Vein Occlusion Study Group. Ophthalmology. 1995;102:1425-1433.
2. Ip MS, et al. Arch Ophthalmol . 2009;127:1101-1114.
3. Brown DM, et al. Ophthalmology . 2010;117:1124-1133.
4. Haller JA, et al. Ophthalmology . 2010;117:1134-1146.
5. Heier JS, et al. Ophthalmology . 2012;119:802-809.
6. Boyer D, et al. Ophthalmology . 2012;119:1024-1032.
7. Brown D, et al . Am J Ophthalmol. 2013;155:429
–437
8. Holz FG, et al. Br J Ophthalmology . 2013;97:278-284.
9. Korobelnik J-F, et al. Ophthalmology.
2013;121(1):202-8
*Timeline excludes Avastin
® and Macugen
® studies
34
Date of Prep March 2015
L.GB.01.2014.4924b
Variables in study design
• Primary outcomes
– Mean change in visual acuity
– Proportion of patients improving by ≥15 letters
– Time to improvement of ≥15 letters
• Inclusion/exclusion criteria
– All retinal vein occlusion or CRVO only
– Non-ischaemic patients only or ischaemic and non-ischaemic patients
• Baseline characteristics including visual acuity
• Duration of disease
35
Date of Prep March 2015
L.GB.01.2014.4924b
Central Vein Occlusion Study (CVOS):
Aims and inclusion/exclusion criteria
Aim
To evaluate efficacy of macular grid photocoagulation in preserving or improving central visual acuity in eyes with macular oedema due to central vein occlusion, and BCVA ≤6/15 (20/50)
Inclusion
CVO of ≥3 months
Confirmed macular oedema involving fovea
VA 5/200 to 20/50 (2/60 to 6/15)
Exclusion
Previous laser photocoagulation for retinal vascular disease of the study eye
Other eye disease that might affect VA
Presence of diabetic retinopathy, branch arterial/vein occlusion, retinal neovascularisation, other retinal vascular disease, vitreous haemorrhage
Presence of peripheral anterior synechia in study eye Phakic, clear media
No improvement to VA before study entry
Intraocular pressure <30 mmHg
Good fundus/FA photography possible
The Central Vein Occlusion Study Group M. Ophthalmology . 1995;102:1425-1433.
36
Date of Prep March 2015
L.GB.01.2014.4924b
CVOS: Baseline characteristics
Characteristics
Number of eyes
Specified characteristics (%)
Age (years)
<60
60 –74
75
Male
White
Smoker
Present
Past
Duration of CRVO
<1 month
<1 year
1 year
Visual acuity
20/20 or better
20/25 –20/40
20/50
–20/100
20/125 –20/200
20/250
–5/200
<5/200
Treated
77
29
45
26
66
92
12
48
0
52
48
0
0
39
36
25
0
The Central Vein Occlusion Study Group M. Ophthalmology . 1995;102:1425-1433.
13
46
1
56
42
0
0
46
35
19
0
Untreated
78
22
55
23
53
96
P-value
–
0.47
0.10
0.38
1.00
0.57
0.60
37
Date of Prep March 2015
L.GB.01.2014.4924b
CVOS: Baseline characteristics (continued)
Characteristics
Disc areas of macula oedema
None
<2
2−<5
5
Unavailable
Disc areas of ischaemia
None
<5
5−<10
10
Unavailable
Treated
0
3
36
61
0
29
35
13
13
10
Untreated
0
3
44
53
1
42
32
10
8
8
P-value
0.63
0.44
The Central Vein Occlusion Study Group M. Ophthalmology . 1995;102:1425-1433.
38
Date of Prep March 2015
L.GB.01.2014.4924b
CVOS: Study design
3-year, multicentre, randomised clinical trial comparing macular grid laser photocoagulation with observation in eyes with macular oedema secondary to CRVO
CRVO patients (N=155) with visual acuity ≤20/50 and
FA evidence of macular oedema involving the fovea
Randomisation
1:1
Treated (n=77) a Untreated (n=78)
Primary outcome: change in visual acuity a Argon laser grid photocoagulation applied according to standard protocol.
The Central Vein Occlusion Study Group M. Ophthalmology . 1995;102:1425-1433.
39
Date of Prep March 2015
L.GB.01.2014.4924b
CVOS: Grid laser provided no improvement in visual acuity at 3 years
Change in visual acuity from baseline*
Letters
15
10
5
30
25
20
0
-5
-10
-15
-20
-25
-30
Treated
Untreated
0 4 8 12 16 20 24 28 32
P value not reported.
Month of follow-up
Horizontal bars = ± 1 standard error of the mean; horizontal line = no change in visual acuity score.
* Subjects with central retinal vein occlusion of 1 year or more
36
The Central Vein Occlusion Study Group M. Ophthalmology . 1995;102:1425-1433.
Lines
3
2
1
6
5
4
0
-1
-2
-3
-4
-5
-6
40
Date of Prep March 2015
L.GB.01.2014.4924b
CVOS: Summary and key messages
Summary
Mean change in BCVA (letters)
(treated patients)
% patients ≥15 letter gain
-6 at 12 months
-4 at 36 months
6 at 12 months
Key messages
• There was angiographic evidence of improvement in macular oedema, but no improvement in visual acuity
• Macular grid photocoagulation is ineffective in improving visual function in patients with CRVO
The Central Vein Occlusion Study Group M. Ophthalmology . 1995;102(10):1425-1433.
41
Date of Prep March 2015
L.GB.01.2014.4924b
Standard Care vs. Corticosteroid for
Retinal Vein Occlusion (SCORE-CRVO):
Aims and inclusion/exclusion criteria
Aim
To compare the efficacy and safety of preservative-free intravitreal triamcinolone vs. observation for vision loss associated with macular oedema secondary to perfused CRVO
Inclusion
Bestcorrected ETDRS visual acuity letter score of ≤73
(approximate Snellen equivalent, 20/40 or worse) and ≥19
(20/400 or better)
Exclusion
Macular oedema not caused by CRVO
Ocular condition where VA would not improve from oedema resolution (e.g. foveal atrophy)
Cataract reducing VA by ≥3 lines
Centre-involved macular oedema secondary to CRVO present on clinical examination
Mean central subfield retinal thickness of 2 OCT fast macular scans, ≥250 μm
Treatment with intravitreal steroids, or peribulbar steroid injection within 6 months of randomisation
History of recent focal/grid macular photocoagulation, panretinal photocoagulation, or anticipated need for panretinal photocoagulation
Conditions to allow adequate fundus photography Prior pars plana vitrectomy
Major actual/anticipated eye surgery (incl. cataracts)
IOP ≥25 mmHg, open-angle glaucoma, steroid-induced
IOP elevation requiring IOP-lowering treatment, or pseudoexfoliation
Aphakia
Ip MS, et al; SCORE Study Research Group. Arch Ophthalmol . 2009;127:1101-1114.
42
Date of Prep March 2015
L.GB.01.2014.4924b
SCORE-CRVO: Baseline characteristics
Characteristic
Participants
Demographic characteristics
Mean (SD) age, y
Min/max
Women
White
Study eye characteristics
Mean (SD) E-EDTRS VA letter score (Snellen equivalent)
73 –59 (20/40–20/63)
58 –49 (20/80–20/100)
48
–19 (20/125–20/400)
Duration of macula oedema (months)
<3
3 –6
7
–12
>12
IOP (mmHg)
IOP-lowering medication
Phakic
Observation, n
(%)
88
69.2 (12.8)
35/93
40 (45)
81 (92)
1 mg, n
(%)
92
4 mg, n
(%)
91
Total
271
67.4 (12.4)
32/88
43 (47)
84 (91)
67.5 (12.0)
27/91
40 (44)
82 (90)
68.0 (12.4)
27/93
123 (45)
247 (91)
52.1 (13.1)
33 (38)
20 (23)
35 (40)
4.2 (3.1)
29 (33)
43 (49)
14 (16)
2 (2)
15.4 (3.2)
9 (10.0)
66 (75)
50.6 (14.9)
33 (36)
19 (21)
40 (43)
4.5 (4.2)
36 (39)
38 (41)
14 (15)
4 (4)
15.3 (3.2)
4 (4.3)
77 (84)
51.0 (14.4)
34 (37)
19 (21)
38 (42)
4.2 (3.6)
40 (44)
34 (37)
15 (16)
2 (2)
15.8 (3.2)
7 (7.7)
76 (84)
51.2 (14.1)
100 (37)
58 (21)
113 (42)
4.3 (3.7)
105 (39)
115 (42)
43 (16)
8 (3)
15.5 (3.2)
20 (7.4)
219 (81)
Ip MS, et al; SCORE Study Research Group. Arch Ophthalmol . 2009;127:1101-1114.
43
Date of Prep March 2015
L.GB.01.2014.4924b
SCORECRVO: Baseline characteristics (cont’d)
Characteristic Observation, n (%)
Other clinical characteristics
Diabetes mellitus
Hypertension
Coronary artery disease
History of cancer
Imaging data, mean (SD)
OCT centre point thickness ( μm)
Total macular volume, mean (SD), mm 3
Area of retinal thickening within the grid, mean SD, DA
Area of retinal haemorrhage within the grid, mean SD, DA
Area of fluorescein haemorrhage within the grid, mean SD, DA
>10 DA of capillary ischaemia in the eye
Mean (SD) non-study eye E-ETDRS VA letter score
22 (25)
70 (80)
20 (23)
14 (16)
695 (208)
10.4 (1.7)
13.0 (4.6)
3.6 (3.0)
11.6 (4.8)
0 (0)
80.8 (15.0)
1 mg, n
(%)
17 (18)
63 (68)
17 (18)
19 (21)
4 mg, n
(%)
23 (25)
64 (70)
19 (21)
25 (27)
Total
62 (23)
197 (73)
56 (21)
58 (21)
643 (226)
10.6 (2.0)
12.2 (4.8)
3.1 (3.2)
10.9 (5.0)
2 (3)
641 (248)
10.0 (2.1)
11.8 (5.1)
3.4 (3.5)
10.4 (5.1)
1 (2)
659 (229)
10.3 (2.0)
12.3 (4.8)
3.4 (3.3)
10.9 (5.0)
3 (2)
81.2 (12.6) 81.5 (10.3) 81.2 (12.7)
DA: disc area; E-ETDRS: electronic Early Treatment Diabetic Retinopathy Study; IOP: intraocular pressure; OCT: optical coherence tomography; SD: standard deviation.
Ip MS, et al; SCORE Study Research Group. Arch Ophthalmol . 2009;127:1101-1114.
44
Date of Prep March 2015
L.GB.01.2014.4924b
SCORE-CRVO: Study design
36-month multicentre, randomised clinical trial comparing intravitreal triamcinolone (Trivaris, a preservative-free formulation*) with observation for macular oedema and CRVO
Adults aged ≥27 years (N=271) with macular oedema secondary to
CRVO with retinal thickness (CPT) ≥250 µm and BCVA of 20/40 to 20/400
Triamcinolone every 4 months
1 mg (n=92) or 4 mg (n=91)
Randomisation
1:1:1
Observation
(n=88)
Baseline to month 12 (N=238) (primary endpoint; visual acuity gain ≥15 letters)
Continued treatment to month 24 (N=151)
Continued treatment to month 36 (N=81)
*Only unlicensed triamcinolone containing preservatives is available. This has been associated with post-injection inflammation.
CPT = centre point thickness; SCORE = Standard Care Versus Corticosteroid for Retinal Vein Occlusion.
45
Ip MS, et al; SCORE Study Research Group. Arch Ophthalmol . 2009;127:1101-1114.
Date of Prep March 2015
L.GB.01.2014.4924b
SCORE-CRVO: Greater visual acuity gains in triamcinolone arms at month 12
Proportion of patients with BCVA gain/loss
Observation (n=73) 1 mg triamcinolone (n=83) 4 mg triamcinolone (n=82)
50
40
50
40
44
30
27 26
30
25 26
20 20
10
11 10
13
8
15
10
7 10
7
5
4 4
5
4
0
5 –9 10 –14
Gain
≥15 a
0
5 –9 10 –14
Loss
≥15 a P values for pairwise comparisons with a gain in visual acuity letter score of 15 or more are: 1 mg triamcinolone vs. observation: P =0.001; 4 mg triamcinolone vs. observation: P =0.001; 4 mg triamcinolone vs. 1 mg triamcinolone: P =0.97.
Ip MS, et al; SCORE Study Research Group. Arch Ophthalmol . 2009;127:1101-1114.
46
Date of Prep March 2015
L.GB.01.2014.4924b
SCORE-CRVO: CPT decreases from baseline shown for all groups
Proportion of patients with retinal thickness (CPT) >500 μm
100
90
80
70
60
50
40
30
20
10
0
Baseline 4 8 12
Months
Observation
1 mg triamcinolone
4 mg triamcinolone
16 20 24
CPT: centre point thickness.
Ip MS, et al; SCORE Study Research Group. Arch Ophthalmol . 2009;127:1101-1114.
47
Date of Prep March 2015
L.GB.01.2014.4924b
SCORE-CRVO: Higher dose of steroid produced more ocular adverse events
Ocular adverse events
Observation n=88, n (%)
Triamcinolone 1 mg n=92, n (%)
Elevated intraocular pressure (IOP) or glaucoma a
IOP-lowering medication 7 (8.0) 18 (19.6)
IOP >35 mmHg
IOP >10 mmHg over baseline
Cataract
1 (1.1)
2 (2.3)
5 (5.4)
15 (16.3)
Lens opacity/progression
Cataract
12 (13.6)
0
20 (21.7)
0
Triamcinolone 4 mg n=91, n (%)
32 (35.2)
8 (8.8)
24 (26.4)
25 (27.5)
4(4.4) a More eyes in the 4-mg group received IOP-lowering medication compared with the 1-mg and observation groups;
P =0.02 for the observation vs. 1 mg comparison; P <0.001, observation vs. 4 mg; and P =0.02, 1 mg vs. 4 mg.
Ip MS, et al; SCORE Study Research Group. Arch Ophthalmol . 2009;127:1101-1114.
48
Date of Prep March 2015
L.GB.01.2014.4924b
SCORE-CRVO: Higher dose of steroid produced more ocular adverse events
Other ocular adverse events
Observation n=88 (%)
Triamcinolone 1 mg n=92 (%)
At least one of the following adverse events
Infectious endophthalmitis 0 0
Non-infectious endophthalmitis
Retinal detachment
Iris neovascularisation or neovascular glaucoma
Retinal neovascularisation
0
0
2 9
4
4 Vitreous haemorrhage
Other ocular surgical procedures
YAG laser capsulotomy
Sector or panretinal scatter photocoagulation
Pars plana vitrectomy
1
5
1
Ip MS, et al; SCORE Study Research Group. Arch Ophthalmol . 2009;127:1101-1114.
0
9
2
2
4
0
0
Triamcinolone 4 mg n=91 (%)
4
2
0
0
0
0
0
3
0
49
Date of Prep March 2015
L.GB.01.2014.4924b
SCORE-CRVO: Summary and key messages
Summary
Mean change in BCVA (letters)
% patients ≥15 letter gain at month 12
-1.2 letters for both 1 mg and 4 mg doses
27% (1 mg triamcinolone)
26% (4 mg triamcinolone)
Number of injections Approximately 2 over 12 months
Retinal thickness (central point thickness) No difference between triamcinolone groups and observation control group
Key messages
• Intravitreal triamcinolone injected every 4 months is superior to observation alone for improving vision in patients with macular oedema secondary to CRVO
• Rates of elevated IOP and cataract were higher in the 4-mg triamcinolone group vs. control
Ip MS, et al; SCORE Study Research Group. Arch Ophthalmol . 2009;127:1101-1114.
50
Date of Prep March 2015
L.GB.01.2014.4924b
Global Evaluation of implaNtable dExamethasone in retinal Vein occlusion with macular edemA (GENEVA):
Aims and inclusion/exclusion criteria
Aim
To evaluate safety and efficacy of dexamethasone intravitreal implant (Ozurdex ®) ) vs. sham in eyes with vision loss due to macular oedema (MO) after branch retinal vein occlusion (BRVO)/central retinal vein occlusion (CRVO)
Inclusion Exclusion
Decreased VA as a result of clinically detectable MO associated with CRVO (6 weeks to 9 months duration) or
BRVO (6 weeks to 12 months duration)
Presence of clinically significant epiretinal membrane, active retinal or optic disc neovascularisation
BCVA 34 to 68 letters (approx 6/60 to 6/150) in study eye;
>34 letters (6/60) in non-study eye
Active or history of choroidal neovascularisation
Retinal thickness in central subfield ≥300 μm in study eye Presence of rubeosis iridis
Haller JA, et al. Ophthalmology . 2010;117:1134-1146.
Active infection, aphakia or anterior-chamber intraocular lens, clinically significant media opacity, glaucoma or current ocular hypertension requiring more than 1 medication to control IOP in the study eye, or a history of steroid-induced IOP increase in either eye
Diabetic retinopathy in either eye
Uncontrolled systemic disease
Current/anticipated use of systemic steroids/anticoagulants
Any ocular condition in the study eye that would prevent a 15-letter improvement in visual acuity
51
Date of Prep March 2015
L.GB.01.2014.4924b
GENEVA: Baseline characteristics
Characteristic
Age (years)
Mean (range)
Sex
Male
Female
Race
White
Black
Asian (excl. Japanese)
Japanese
Hispanic
Other
Iris colour
Dark
Light
Diagnosis in study eye
BRVO
CRVO
Duration of macula oedema
Mean duration (range)
<90 days
90 –179 days
180 –269 days
270 days
Mean baseline VA, letters ±SD (Snellen equivalent)
Haller JA, et al. Ophthalmology . 2010;117:1134-1146.
DEX implant 0.7 mg (n=427)
64.7 (33 –90)
217 (50.8%)
210 (49.2%)
321 (75.2%)
15 (3.5%)
38 (8.9%)
0
37 (8.7%)
16 (3.7%)
241 (56.4%)
186 (43.6%)
291 (68.1%)
136 (31.9%)
157.6 (19 –374)
70 (16.4%)
219 (51.3%)
93 (21.8%)
45 (10.5%)
54.3
±9.93 (20/80)
DEX implant
0.35 mg
(n=414)
64.9 (31 –96)
220 (53.1%)
194 (46.9%)
312 (75.4%)
14 (3.4%)
36 (8.7%)
2 (0.5%)
29 (7.0%)
21 (5.1%)
244 (58.9%)
170 (41.1%)
260 (62.8%)
154 (37.2%)
153.0 (49 –944)
76 (18.1%)
218 (52.7%)
89 (21.5%)
32 (7.7%)
53.9
±10.41
(20/80)
Sham (n=426) Amonggroup
P-value
0.453
63.9 (31 –91)
0.268
240 (56.3%)
186 (43.7%)
0.970
318 (74.6%)
20 (4.7%)
44 (10.3%)
1 (0.2%)
25 (5.9%)
18 (4.2%)
0.195
265 (62.5%)
159 (37.5%)
0.264
279 (65.5%)
147 (34.5%)
0.923
156.1 (19 –374)
65 (15.3%)
220 (51.6%)
99 (23.2%)
42 (9.9%)
54.8
±9.86
(20/80)
NS
52
Date of Prep March 2015
L.GB.01.2014.4924b
GENEVA: Baseline characteristics (continued)
Characteristic
Mean baseline retinal thickness ( μm±SD)
Prior laser photocoagulation
BRVO
CRVO
Other procedures for RVO
Haemodilution
Intraocular injection
Lens status
Phakic
Pseudophakic
Diabetes mellitus
Hypertension
Coronary artery disease
IOP-lowering medication use at baseline
DEX implant 0.7 mg (n=427)
562 ±188
41 (10%)
37 (90%)
4 (10%)
DEX implant
0.35 mg
(n=414)
555 ±204
Sham (n=426)
539 ±186
44 (11%)
40 (91%)
4 (9%)
40 (9%)
36 (90%)
4 (10%)
1 (0.2%)
0
373 (88%)
53 (12%)
64 (15%)
264 (62%)
55 (13%)
27 (6%)
1 (0.2%)
1 (0.2%)
362 (87%)
52 (13%)
57 (14%)
264 (64%)
49 (12%)
24 (6%)
2 (0.5%)
1 (0.2%)
387 (91%)
39 (9%)
63 (15%)
273 (64%)
38 (9%)
16 (4%)
Amonggroup
P-value
NS
0.814
0.208
0.866
0.761
0.165
0.210
Ischaemic (perfused disease)
Patients with CRVO were not screened for non-ischaemic or ischaemic disease. The relatively good vision (20/200) of patients at baseline suggests that most patients had non-ischaemic disease, but the development of neovascularisation in 2.6% of sham patients suggests that at least some patients had ischaemic disease
BRVO: branch retinal vein occlusion; CRVO: central retinal vein occlusion; DEX implant: dexamethasone intravitreal implant (OZURDEX, Allergan
Inc., Irvine, CA); IOP: intraocular pressure; NS: not significant; RVO: retinal vein occlusion; SD: standard deviation.
53
Haller JA, et al. Ophthalmology . 2010;117:1134-1146.
Date of Prep March 2015
L.GB.01.2014.4924b
GENEVA: Study design
12-month, phase 3, multicentre, double-masked, trial of treatment with dexamethasone intravitreal implant (DEX) for macular oedema with RVO
Patients (N=1,267) aged ≥18 years with decreased visual acuity due to macular oedema secondary to RVO
Randomisation
1:1:1
DEX implant 0.7 mg
(n=427)
DEX implant 0.35 mg
(n=414)
Sham
(n=426)
Single DEX implant or sham injection at Day 0 (masked treatment) 1
Open-label treatment to month 12 (primary endpoint; safety) 2
At day 180, n = 997
1. Haller JA, et al. Ophthalmology . 2010;117:1134-1146.
2. Haller JA, et al. Ophthalmology . 2011;118:2453-2460.
54
Date of Prep March 2015
L.GB.01.2014.4924b
GENEVA: Mean number of letters gained/lost at 180/360 days
4
2
0
-2
-4
0
12
10
8
6
Dex/dex (all eyes)
Sham/dex (all eyes)
Dex/dex (CRVO)
Sham/dex (CRVO)
30 60 90 120 150 180
Days
210 240 270 300 330 360
Dexamethasone implant or sham
Dexamethasone implant
Masked study Open-label extension
Haller JA, et al. Ophthalmology . 2010;117:1134-1146;
Haller JA, et al. Ophthalmology . 2011;118:2453-2460.
55
Figure adapted from Haller JA, et al. Ophthalmology . 2011;118:2453-2460.
Date of Prep March 2015
L.GB.01.2014.4924b
GENEVA: Mean number of letters gained/lost at 180/360 days
4
2
0
-2
-4
0
12
10
8
6
Dex/dex (CRVO)
Sham/dex (CRVO)
30 60 90 120 150 180
Days
210 240 270 300 330 360
Dexamethasone implant or sham
Dexamethasone implant
Masked study Open-label extension
Haller JA, et al. Ophthalmology . 2010;117:1134-1146.
Haller JA, et al. Ophthalmology . 2011;118:2453-2460.
56
Figure adapted from Haller JA, et al. Ophthalmology . 2011;118:2453-2460.
Date of Prep March 2015
L.GB.01.2014.4924b
GENEVA: Elevated intraocular pressure and cataracts
Ocular adverse events
Sham n=423 (%)
Dexamethasone implant 0.35 mg n=412 (%)
Dexamethasone implant 0.7mg n=421 (%)
Elevated intraocular pressure (IOP) or glaucoma
IOP-lowering medication (at day 180) 6/423 (1.4) 103/239 (25) 109/341 (25.9)
P-value
IOP >35 mmHg (at day 60)* (0) (4) (3.5)
IOP >25 mmHg (at day 60)* (0) (15)*
IOP >10 mmHg over baseline (at day 60)*
Cataract (at day 360)
0
5/88 (5.7)
(15)*
56/283 (19.8)
(15)*
(15)*
90/302 (29.8) p<0.001 vs sham p<0.001 vs sham
* Intraocular pressure peaked at day 60 and reverted to near-baseline values by day 180
Haller JA, et al. Ophthalmology . 2010;117(6):1134-1146.
57
Date of Prep March 2015
L.GB.01.2014.4924b
GENEVA: Higher rates of treatment-related adverse events in dexamethasone (DEX)-treated patients
Retreated
DEX
Implant
0.7/0.7
(n=341) a,b
Retreated
DEX
Implant
0.35/0.7
(n=329)
216
(63.3%)
205
(62.3%)
Ocular adverse events a treatment
Pvalue
Single
DEX
Single
DEX
DEX
Implant
Sham/0.7
b
(n=327)
Implant
0.7/None
(n=80)
Implant
0.35/None
(n=83)
162
(49.5%)
Untreated
Sham/None
(n=96)
P-value
<0.001
42 (52.5%) 40 (48.2%) 10 (10.4%) <0.001
a In the group receiving two 0.7-mg dexamethasone implants (n=341), a ≥10-mmHg lOP increase was seen in 12.6% after the first treatment, and 15.4% after the second (4 serious adverse events in patients treated with dexamethasone implant were considered to be related to treatment (1 retinal detachment; 3 elevated lOPs) b Cataract progression occurrence was 29.8% for patients who received two 0.7-mg dexamethasone implants vs. 5.7% of sham-treated eyes
Haller JA, et al. Ophthalmology . 2011;118:2453-2460.
58
Date of Prep March 2015
L.GB.01.2014.4924b
GENEVA: Conclusions
• Although patients were not screened, baseline visual acuity suggests that most had non-ischaemic disease 1
• Dexamethasone implant produced greater and more rapid improvements in vision than sham 1,2
• BCVA was at a maximum at 60 days, and reverted to baseline by day 180 1
• There was an increase in IOP despite treatment 1,2
• There were more cataract adverse events in the dexamethasone implant-treated group compared with sham 2
• Treatment delay resulted in worse visual acuity outcomes
1. Haller JA, et al. Ophthalmology . 2010;117:1134-1146.
2. Haller JA, et al. Ophthalmology . 2011;118:2453-2460.
59
Date of Prep March 2015
L.GB.01.2014.4924b
GENEVA: Summary and key messages
Summary
Mean change in BCVA (letters)
(0.7mg dose)
2.3 vs. baseline at 360 days
(peak difference 7.7 letters at 240 days,
60 days after 2 nd injection)
% patients ≥15 letter gain
(0.7mg dose)
24% at 360 days (0.7 mg dose)
Peak 32% at day 240, 60 days after
2 nd dose
2 Mean number of injections over 12 months
Mean change in retinal thickness (central retinal thickness) (0.7mg dose)
-166 μm at 360 days
Key messages
• Dexamethasone implant has rapid, small, short-lived effect on VA
• Cataracts: 29.8% in 12 months in patients with 2 dexamethasone implant treatments vs 10.5% in those with 1 treatment
• 32.8% of eyes treated twice with dexamethasone had >10 mmHg rise in IOP
Haller JA, et al. Ophthalmology . 2010;117:1134-1146.
Haller JA, et al. Ophthalmology . 2011;118:2453-2460.
IOP: intraocular pressure; ns: non-significant; VA: visual acuity.
60
Date of Prep March 2015
L.GB.01.2014.4924b
Steroid therapy for macular oedema secondary to CRVO
• Intravitreal steroids were the first drugs to be used for the medical therapy of proliferative, oedematous, and neovascular diseases
• Systemic and local adverse effects include:
– Cataract
– Secondary ocular hypertension/increased IOP/glaucoma
– Post-injection sterile and/or infectious endophthalmitis
• Limited duration of intraocular availability and effect
Jonas JB, Lam DSC. Asia-Pac J Ophthalmol . 2012;1:355-363.
61
Date of Prep March 2015
L.GB.01.2014.4924b
Clinical trials of ranibizumab in CRVO
• CRUISE
• HORIZON
Central Retinal Vein OcclUsIon Study:
Evaluation of Efficacy and Safety (CRUISE):
Aims and inclusion/exclusion criteria
Aim
To assess efficacy and safety of intraocular injections of 0.3 mg or 0.5 mg ranibizumab in patients with macular oedema after central retinal vein occlusion
Inclusion Exclusion
Macular oedema secondary to CRVO diagnosed
<12 months before study initiation
Brisk relative afferent pupillary defect
(i.e. obvious and unequivocal)
>10-letter improvement in BCVA between screening and day 0
BCVA 6/12 (20/40) to 6/100 (20/320) History of radial optic neurotomy or sheathotomy
Recent intraocular steroid use in study eye
History or presence of wet or dry AMD
Mean retinal thickness (central subfield)
≥250 μm (2 OCT measurements)
Evidence of diabetic retinopathy
Recent stroke or MI
Recent anti-VEGF treatment
Brown DM, et al. Ophthalmology . 2010;117:1124-1133.
Campochiaro PA, et al. Ophthalmology . 2011;118:2041-2049.
63
Date of Prep March 2015
L.GB.01.2014.4924b
CRUISE: Baseline characteristics
Characteristic
Age (years)
Mean (SD)
Sex
Male
Female
Race
White
Black
Other
Unavailable
Study eye characteristics
Month from RVO diagnosis to screening
Mean (SD)
Median
Range
Distribution, n (%)
≤3
>3 to ≤6
>6 to ≤9
>9 to ≤12
>12
BCVA
EDTRS letter score
Mean (SD)
Range
Distribution, n (%)
<34
35 –54
55
Approximate Snellen equivalent
.
Brown DM, et al. Ophthalmology . 2010;117:1124-1133.
Sham (n=130)
65.4 (13.1)
72 (55.4)
58 (44.6)
113 (86.9)
8 (6.2)
7 (5.4)
3 (2.3)
2.9 (2.9)
2
0 –14
91 (70.0)
27 (20.8)
4 (3.1)
7 (5.4)
1 (0.8)
49.2 (14.7)
16
–71
26 (20.0)
49 (37.7)
55 (42.3)
20/100
Ranibizumab 0.3 mg
(n=132)
69.7 (11.6)
71 (53.8)
61 (46.2)
108 (81.8)
16 (12.1)
3 (2.3)
5 (3.8)
Ranibizumab 0.5 mg
(n=130)
67.6 (12.4)
80 (61.5)
50 (38.5)
108 (83.1)
10 (7.7)
7 (5.4)
5 (3.8)
3.6 (3.2)
2
0 –12
87 (65.9)
18 (13.6)
16 (12.1)
11 (8.3)
0
3.3 (3.7)
2
0 –27
94 (72.3)
17 (13.1)
10 (7.7)
6 (4.6)
3 (2.3)
47.4 (14.8)
9
–72
33 (25.0)
46 (34.8)
53 (40.2)
20/100
48.1 (14.6)
21
–73
30 (23.1)
50 (38.5)
50 (38.5)
20/100
64
Date of Prep March 2015
L.GB.01.2014.4924b
CRUISE: Baseline characteristics (continued)
Characteristic
IOP (mmHg), mean (SD)
IOP-lowering medication, n (%)
Phakic eye, n (%)
Imaging data
CFT (
μm), mean (SD)
Total macular volume (mm 3 ), mean (SD)
Total area of retinal haemorrhage, central subfield (DA), mean (SD)
Area of fluorescein leakage within grid (DA), median
>10 DA of capillary ischaemia (%)
Fellow eye characteristics
Fellow eye BCVA (ETDRS letters), mean (SD)
Fellow eye vision compared with study eye, n (%)
Better
Worse
Same
Sham (n=130)
15.1 (3.1)
13 (10.0)
88 (80.7)
687.0 (237.6)
10.700 (2.303)
0.080 (0.113)
15
0
78.9 (18.6)
117 (90.0)
8 (6.2)
5 (3.8)
Ranibizumab
0.3 mg (n=132)
14.9 (3.3)
18 (13.6)
84 (75.0)
679.9 (242.4)
10.748 (2.380)
0.093 (0.117)
15
0
80.0 (12.5)
123 (93.2)
3 (2.3)
6 (4.5)
Ranibizumab
0.5 mg (n=130)
15.1 (3.4)
22 (16.9)
83 (72.8)
688.7 (253.1)
10.308 (2.033)
0.093 (0.117)
14
2
78.8 (17.4)
120 (92.3)
7 (5.4)
3 (2.3)
CFT: central foveal thickness; DA: disc areas; EDTRS: Early Treatment Diabetic Retinopathy Study: IOP: intraocular pressure; RVO retinal vein occlusion; SD: standard deviation.
.
Brown DM, et al. Ophthalmology . 2010;117:1124-1133.
65
Date of Prep March 2015
L.GB.01.2014.4924b
CRUISE: Study design
12-month, phase 3, prospective, randomised, double-masked, multicentre trial comparing
0.3 mg or 0.5 mg ranibizumab with sham in CRVO with macular oedema
Patients (N=392) aged ≥18 years with macular oedema secondary to CRVO with retinal thickness (CFT) ≥250 µm and ETDRS BCVA of 6/12 (20/40) to 6/100 (20/320)
Ranibizumab 0.3 mg
(n=132)
Randomisation
1:1:1
Ranibizumab 0.5 mg
(n=130)
Sham a
(n=130)
Monthly treatment to month 6 (N=363) (primary endpoint; mean change from baseline BCVA) 1
PRN treatment to month 12 (N=349) 2 a After 6 months, all patients with study eye BCVA ≤20/40 or central foveal thickness (CFT) ≥250 µm were to receive ranibizumab.
1. Brown DM, et al. Ophthalmology . 2010;117:1124-1133.
2. Campochiaro PA, et al. Ophthalmology . 2011;118:2041-2049.
66
Date of Prep March 2015
L.GB.01.2014.4924b
CRUISE: Ranibizumab significantly improved
BCVA at 6 and 12 months
10
8
2
0
-2
6
4
18
16
14
12
7 day
Mean change in BCVA a
2
Day 0 –month 5
4 monthly treatment 1
At 6 months patients with
BCVA ≤6/12 or retinal thickness (CFT) ≥250 µm to receive ranibizumab.
+12.7
b
14.1 letters difference
+0.8
6
+14.9
b
Sham/0.5 mg (n=130)
0.3 mg Ranibizumab (n=132)
0.5 mg Ranibizumab (n=130)
Mean No. PRN phase injections
Ranibizumab 0.3 mg: 3.8
Ranibizumab 0.5 mg: 3.3
Sham/0.5 ranibizumab: 3.7
12 8
Months 6 –11
10
PRN treatment 2
+13.9
c
+13.9
c
6.6 letters difference
+7.3
Month a After 6 months, all patients with study eye BCVA ≤20/40 or central foveal thickness (CFT) ≥250 µm were to receive ranibizumab.
b P <0.0001vs sham, c P <0.001 vs sham/0.5 mg.
Vertical bars are
±1 standard error of the mean.
Figure adapted from Campochiaro PA, et al. Ophthalmology . 2011.
Last observation carried forward method used to impute missing values.
Brown DM, et al. Ophthalmology . 2010;117:1124-1133.
Campochiaro PA, et al. Ophthalmology . 2011;118:2041-2049.
67
Date of Prep March 2015
L.GB.01.2014.4924b
CRUISE: Ranibizumab significantly reduced retinal thickness at 6 months
50
0
0
Mean change in retinal thickness (CFT) a
Day 0 –month 5 monthly treatment 1
Month
Months 6
–11
PRN treatment 2
7 days 2 4 6 8 10 12
-100
-200
-167.7
1
Mean No. PRN phase injections
Ranibizumab 0.3 mg: 3.8
Ranibizumab 0.5 mg: 3.3
Sham/0.5 ranibizumab: 3.7
-300
-400
-433.7
1,*
-452.3
1*
-427.2
2
-452.8
2
-462.1
2
-500
At 6 months patients with
BCVA ≤6/12 or central foveal thickness (CFT) ≥250 µm to receive ranibizumab.
Sham/0.5 mg (n=129)
0.3 mg Ranibizumab (n=131)
0.5 mg Ranibizumab (n=130)
* P <0.0001 vs. sham.
Vertical bars are ±1 standard error of the mean.
Figure adapted from Campochiaro PA, et al. Ophthalmology . 2011.
Last observation carried forward method used to impute missing values
CFT = central foveal thickness.
1. Brown DM, et al. Ophthalmology . 2010;117:1124-1133.
2. Campochiaro PA, et al. Ophthalmology . 2011;118:2041-2049.
68
Date of Prep March 2015
L.GB.01.2014.4924b
CRUISE: Main ocular adverse events
Sham a
Day 0 –
Month 6
(n=129)
Any ocular inflammation
Cataract
5 (3.9%)
0
Iris neovascularisation 9 (7.0%)
Retinal tear 0
Vitreous haemorrhage 9 (7.0%) c
Sham/
0.5 mg b
Months 6 –12
(n=110)
Ranibizumab
0.3 mg
Day 0 – Month 12
(n=132)
Ranibizumab
0.5 mg
Day 0 – Month 12
(n=129)
2 (1.8%) 3 (2.3%) 2 (1.6%)
2 (1.8%) c
2 (1.8%)
2 (1.8%) c
2 (1.8%) c
5 (3.8%)
2 (1.5%)
0
7 (5.3%)
9 (7.0%)
5 (3.9%)
2 (1.6%)
7 (5.4%) a Outcomes during 6-month treatment period for safety-evaluable shamgroup patients (≥1 sham injection).
b Outcomes during 6-month observation period for safetyevaluable sham/0.5 mg group patients (≥1 0.5 mg ranibizumab injection).
c One event reported as serious.
Campochiaro PA, et al. Ophthalmology . 2011;118:2041-2049.
69
Date of Prep March 2015
L.GB.01.2014.4924b
CRUISE: Non-ocular adverse events potentially related to anti-VEGF treatment
Sham a
Day 0 – Month 6
(n=129)
Sham/0.5 mg b
Months 6 –12
(n=110)
Ranibizumab 0.3 mg
Day 0 – Month 12
(n=132)
Ranibizumab 0.5 mg
Day 0 – Month 12
(n=129)
Serious adverse events potentially related to VEGF inhibition, n (%)
Haemorrhagic shock 0 0 0 0
Ischaemic stroke
Transient ischaemic attack
Myocardial infarction
0
0
1 (0.8)
0
0
0
0
1 (0.8)
1 (0.8)
1 (0.8)
1 (0.8) c
1 (0.8)
1 (0.8) c Angina pectoris 0 0 0
Hypertension
Non-ocular haemorrhage, other
Proteinuria
APTC ATEs, n (%)
Vascular death
Death from unknown cause
Non-fatal MI
1 (0.8)
0
0
1 (0.8)
0
0
1 (0.8)
0
0
0
0
0
0
0
0
0
0
1 (0.8)
0
0
1 (0.8)
0
0
0
3 (2.3)
0
1 (0.8)
1 (0.8)
Non-fatal haemorrhagic stroke 0 0 0 0
Non-fatal ischaemic stroke 0 0 0 1 (0.8) a Outcomes during 6-month treatment period for safety-evaluable shamgroup patients (≥1 sham injection).
b Outcomes during 6-month observation period for safetyevaluable sham/0.5 mg group patients (≥1 0.5 mg ranibizumab injection).
c Both events occurred in the same patient .
Campochiaro PA, et al. Ophthalmology . 2011;118(10):2041-2049.
70
Date of Prep March 2015
L.GB.01.2014.4924b
CRUISE: Conclusions
• Ranibizumab groups
– Ranibizumab monthly for 6 months provided improvements in visual acuity and macular oedema following CRVO 1
– In the PRN treatment period, months 6 –11, visual and anatomic benefits achieved by monthly ranibizumab were maintained 2
• Sham/0.5 mg ranibizumab group
– After sham for 6 months, ranibizumab PRN for 6 months resulted in CFT reduction similar to 0.3 mg ranibizumab monthly 2
– BCVA improved, but less than in the ranibizumab groups 2
•
Ocular safety event rates were low in all treatment groups 2
• No evidence that ischaemic patients respond: few patients with
>10 disc areas oedema included, and relative afferent pupillary test likely to exclude ischaemia 1,2
1. Brown DM, et al. Ophthalmology . 2010;117:1124-1133.
2. Campochiaro PA, et al. Ophthalmology . 2011;118:2041-2049.
71
Date of Prep March 2015
L.GB.01.2014.4924b
CRUISE: Summary and key messages
Summary
Mean change in BCVA (letters) 13.9 at 12 months (0.3 and 0.5 mg groups) 1
% patients ≥15 letter gain
Mean number of injections over 12 months
(6 in initial protocol then PRN)
Mean change in retinal thickness (central retinal thickness)
Key messages
47.7% (0.5 mg dose) 1
9.3 (0.5 mg dose)
-462 μm (0.5 mg dose) 1
• Anti-VEGF treatment achieved significant improvement in BCVA at 12 months vs. sham 1
• A 6-month delay to anti-VEGF treatment resulted in reduced BCVA improvement vs.
no delay 1,2
• Ischaemic patients effectively excluded (RAPD test exclusion) 2
1. Campochiaro PA, et al. Ophthalmology . 2011;118:2041-2049.
2. Brown DM, et al. Ophthalmology . 2010;117:1124-1133.
72
Date of Prep March 2015
L.GB.01.2014.4924b
HORIZON: Aim and inclusion/exclusion criteria
Aim
To assess long-term safety and efficacy of intraocular ranibizumab injections in patients with macular oedema after retinal vein occlusion
Inclusion Exclusion
Patients with either branch or retinal vein occlusion who completed CRUISE (CRVO) or
BRAVO (BRVO) studies
Intraocular surgery within 1 month of study entry
Use of intravenous bevacizumab in either eye
Concurrent use of systemic anti-VEGF agents
Use of any non-FDA-approved treatments for treatment of study eye
Macular oedema in the study eye due to causes other than RVO (such as diabetic retinopathy)
Heier JS, et al. Ophthalmology . 2012;119:802-809.
73
Date of Prep March 2015
L.GB.01.2014.4924b
HORIZON: Study design
12-month, open-label, single-arm, non-randomised, multicentre, evaluation of ranibizumab PRN for RVO with macular oedema: extension of BRAVO and CRUISE trials (patients originally on sham/0.3mg 0.3/0.5mg or 0.5/0.5mg ranibizumab)
Adults (N=608) with macular oedema secondary to BRVO or
CRVO who completed the BRAVO or CRUISE trials
BRAVO (n=304) CRUISE (n=304)
Sham/ranibizumab
0.5 mg (n=97)
Ranibizumab
0.3/0.5 mg (n=103)
Ranibizumab
0.5 mg (n=104)
Sham/ranibizumab
0.5 mg (n=98)
Ranibizumab
0.3/0.5 mg (n=107)
Ranibizumab
0.5 mg (n=99)
Ranibizumab
0.5 mg PRN
Quarterly follow-up for 12 months (primary endpoints: safety and efficacy of ranibizumab)
Heier JS, et al. Ophthalmology . 2012;119(4):802-809.
74
Date of Prep March 2015
L.GB.01.2014.4924b
HORIZON: PRN dosing phase resulted in lost visual acuity gains
25
20
15
10
5
0
-5
Baseline
Mean change BCVA (CRUISE)
Mean No. PRN phase injections
Ranibizumab 0.3 mg: 3.5
Ranibizumab 0.5 mg: 3.8
Sham/0.5 ranibizumab: 2.9
CRUISE HORIZON CRVO
+16.2
a
+14.9
a
+9.4
a
M12 3 6
Month
9
+12.0
a
+8.2
a
+7.6
a
12
SEM = standard error of the mean; vertical bars are ± 1 SEM.
a Includes patients with data available at that time point and CRUISE baseline.
Heier JS, et al. Ophthalmology . 2012;119:802-809.
0.5 mg Ranibizumab
0.3/0.5 mg Ranibizumab
Sham/0.5 mg
75
Date of Prep March 2015
L.GB.01.2014.4924b
HORIZON: PRN dosing phase did not maintain retinal thickness (CFT) reductions
Mean change in retinal thickness (CFT), CRUISE arm
CRUISE HORIZON
Month
Mean No. PRN phase injections
Ranibizumab 0.3 mg: 3.5
Ranibizumab 0.5 mg: 3.8
Sham/0.5 ranibizumab: 2.9
CRUISE baseline M12 3 6 9 12 50
0
-50
-100
-150
-200
-250
-300
-350
-400
-450
-484.6
a
-459.5
a
-481.4
a
-412.2
a
-370.9
a
-418.7
a
SEM = standard error of the mean; vertical bars are ± 1 SEM.
a Includes patients with data available at that time point and CRUISE baseline.
CFT = central foveal thickness
0.5 mg Ranibizumab
0.3/0.5 mg Ranibizumab
Sham/0.5 mg
Heier JS et al. Ophthalmology . 2012;119:802-809.
76
Date of Prep March 2015
L.GB.01.2014.4924b
HORIZON: ocular and non-ocular adverse events
(CRUISE)
Most commonly-reported ocular adverse events at 12 months
CRUISE
Sham/
0.5 mg
(n=60)
0.3/
0.5 mg
(n=70)
0.5 mg
(n=51)
Retinal haemorrhage 18.8% 19.6% 27.3%
Conjunctival haemorrhage
Increased IOP
15.6%
–
15.0%
1
(0.9%)
16.2%
–
• No imbalance seen in frequency of adverse events potentially related to systemic anti-VEGF inhibition
Heier JS, et al. Ophthalmology . 2012;119:802-809.
77
Date of Prep March 2015
L.GB.01.2014.4924b
HORIZON: Other adverse events
(extension of CRUISE)
Any adverse event (AE)
AE that led to discontinuation
Cataract, total
Serious adverse events (SAEs)
Key SAEs
Amaurosis fugax
Cataract
Cystoid macular oedema
Endophthalmitis
Macular oedema
Macular ischaemia
Ischaemic optic neuropathy
Retinal vein occlusion
Visual acuity reduced
Visual acuity reduced transiently
Vitreous haemorrhage
Heier JS, et al. Ophthalmology . 2012;119(4):802-809.
Sham/0.5 mg
(n=96), n (%)
60 (62.5)
0
3 (3.1)
5 (5.2)
Patients from CRUISE
Ranibizumab
0.3/0.5 mg
(n=107), n (%)
67 (62.6)
2 (1.9)
6 (5.6)
10 (9.3)
Ranibizumab
0.5 mg
(n=99), n (%)
66 (66.7)
2 (2.0)
5 (5.1)
3 (3.0)
1 (1.0)
0
0
0
3 (3.1)
0
0
0
0
0
1 (1.0)
0
1 (0.9)
1 (0.9)
2 (1.9)
2 (1.9)
0
1 (0.9)
0
2 (1.9)
1 (0.9)
0
2 (2.0)
0
0
0
1 (1.0)
0
0
0
0
0
0
78
Date of Prep March 2015
L.GB.01.2014.4924b
HORIZON: Conclusions
• Mean change from baseline (end of CRUISE study) BCVA
(ETDRS letters) was:
– Sham/0.5 mg ranibizumab -4.2
– 0.3/0.5 mg ranibizumab -5.2
– 0.5/0.5 mg ranibizumab -4.1
• Reduced follow-up (quarterly)/fewer injections resulted in declining visual acuity vs. more frequent monitoring/treatment
• May need to see/treat patients more frequently
•
CRVO patients treated with ranibizumab 0.5 mg PRN may require more frequent follow-up than every 3 months
• No new safety events were identified with long-term use of ranibizumab
Heier JS, et al. Ophthalmology . 2012;119:802-809.
79
Date of Prep March 2015
L.GB.01.2014.4924b
HORIZON: Summary and key messages
Summary
Mean change in BCVA (letters)
-4.1 (CRVO 0.5 mg dose 12 months after completion of CRUISE; 6 months fixed monthly treatment then PRN to month 24)
% patients ≥15 letter gain
Mean number of injections (over second 12 months)
45% (0.5 mg dose)
Approximately 2
Mean change in retinal thickness
(central retinal thickness)
CRVO patients
-371 μm from CRUISE baseline
68 μm from HORIZON baseline
Key messages
• Long-term use of ranibizumab well-tolerated
• Reduced frequency of injections in second year of treatment (vs. monthly treatment) associated with worse visual and anatomical outcomes
• Clear differences in outcomes for BRVO vs. CRVO patients
• CRVO patients required frequent follow-up and continued ranibizumab to control oedema
• Open-label non-randomised design is important limitation
• Ischaemic patients effectively excluded
Heier JS, et al. Ophthalmology . 2012;119:802-809.
80
Date of Prep March 2015
L.GB.01.2014.4924b
Bevacizumab in CRVO
• Pan-American Collaborative Retina Study Group trial 1
– Retrospective, 1.25 and 2.5 mg doses
– Largest bevacizumab study, N=86
– Mean number of injections, 7−8 over 24 months
– LOGMAR BCVA improvement 0.27 (2.5 mg) to 0.35
(1.25 mg) units (12 –17 letters)
– 57% gained ≥15 letters over 24 months
• No large randomised controlled trial data
– Low quality evidence
• Unlicensed product
1. Wu L, et al. Retina 2010:30:1002-1011.
81
Date of Prep March 2015
L.GB.01.2014.4924b
Aflibercept development and clinical experience in CRVO
Aflibercept: Specifically designed to block members of the VEGF family 1-3
• Fully human fusion protein 1
– Human VEGF-R1 and VEGF-R2 domains and human IgG1 Fc
• Traps all VEGF-A isoforms and PlGF 1,2
• Higher affinity than native receptors 2
• Formulated for intravitreal injection 3
– Iso-osmotic solution
– Highly purified
Fc: fragment crystallisable/constant region; K
D
: dissociation constant;
PlGF: placental growth factor; VEGF-R1: vascular endothelial growth factor-receptor 1; VEGF-R2: vascular endothelial growth factor-receptor 2
1. Holash J, et al. Proc Natl Acad Sci USA.
2002;99:11393-11398.
2. Dixon JA , et al. Expert Opin Investig Drugs.
2009;18:1573-1580.
3. EYLEA SmPC
Aflibercept development and structure
K
VEGF-R1
D
10 –30 pM
Kinase
K
D
VEGF-R2
100 –300 pM
Amino acids
Kinase
Aflibercept
K
D
<1 pM
IgG1 Fc
Cell membrane
Receptor tyrosine kinases
Figure adapted from Dixon JA, et al.
Expert Opin Investig Drugs. 2009.
2
83
Date of Prep March 2015
L.GB.01.2014.4924b
Mathematical model of comparative biological activity
• Aflibercept 1.15 mg at 79 days ≈ ranibizumab 0.5 mg at 30 days a
• Aflibercept 2 mg at 83 days ≈ ranibizumab 0.5 mg at 30 days b
• Aflibercept 4 mg at 87 days ≈ ranibizumab 0.5 mg at 30 days a
10
5
0
30
25
20
15
Ranibizumab
0.5 mg
Aflibercept
1.15 mg 2 mg b 4 mg
0 20 30 40 60 80 87 100 120
Time (days) a Estimated biological activity.
b Extrapolated.
Stewart MW, Rosenfeld PJ.
Br J Ophthalmol. 2008;92:667-668.
84
Date of Prep March 2015
L.GB.01.2014.4924b
Pharmacokinetics of aflibercept
• After intravitreal administration, mean plasma C max
– 0.02 μg/mL
– Undetectable at 2 weeks
– >100 x lower than aflibercept concentration needed to half maximally bind systemic VEGF
• Systemic pharmacodynamic effects such as blood pressure changes are therefore unlikely
• Accumulation of aflibercept does not occur with repeated
4-weekly doses
• Free and bound aflibercept thought to be cleared by proteolytic catabolism
Eylea SmPC 2015.
85
Date of Prep March 2015
L.GB.01.2014.4924b
Aflibercept mechanism of action
• VEGF-A and PlGF can act as vascular permeability factors for endothelial cells, resulting in neovascularisation and macular oedema 1,2
• Aflibercept acts as a soluble decoy receptor that binds VEGF-A and
PlGF, and so can inhibit binding and activation of VEGF receptors 3,4
OCT demonstrating RVO and macular oedema. Image courtesy of Jeffrey S. Heier MD.
OCT = optical coherence tomography.
1. Keane PA, et al.
J Ophthalmol. 2012;2012:483034.
2. De Falco S. Exp Mol Medicine.
2012;44(1):1-9.
3. Rudge JS, et al. In: Figg WD, Folkman J, editors, Angiogenesis.
New York: Springer; 2008.
4. Holash J, et al. PNAS USA. 2002;99:11393-11398.
86
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS and GALILEO
70 Centres 1
189 Patients
63 Centres 2
177 Patients
COPERNICUS
Canada
USA
COPERNICUS
Colombia
GALILEO
Austria
France
Germany
Hungary
Italy
Latvia
COPERNICUS
India
COPERNICUS
Israel
GALILEO
Australia
Japan
Singapore
South Korea
1. Brown DM, et al. Am J Ophthalmol . 2013;155:429-437.
2. Holz FG, et al. Br J Ophthalmology . 2013;97:278-284.
88
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS:
Aims and inclusion/exclusion criteria
Aim
To evaluate intravitreal aflibercept for patients with macular oedema secondary to CRVO
Inclusion
Centre involved macular oedema secondary to
CRVO diagnosed ≤9 months before study initiation
Retinal thickness (central subfield) ≥250 μm on
OCT
Exclusion
Previous treatment with antiangiogenic drugs, panretinal or macular laser photocoagulation
Ocular disorders that could confound interpretation of study results
Recent use of intraocular/periocular steroids
Iris neovascularisation, vitreous haemorrhage, traction retinal detachment or preretinal fibrosis involving macula
History or presence of age-related macular degeneration (dry or wet) significantly affecting central vision; diabetic macular oedema/diabetic retinopathy
Infectious blepharitis, keratitis, scleritis or conjunctivitis
Brown DM, et al. Am J Ophthalmol . 2013;155:429-437.
89
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: Baseline characteristics
Characteristic Monthly aflibercept aflibercept PRN
(n=114)
Sham aflibercept
PRN (n=73)
Age (years)
Mean (SD) (range) 65.5 (13.57) 67.5 (14.29)
Sex
Male
Female
Race
White
Black
Asian
Other
Geographic region, n (%)
North America
Rest of world
Visual acuity (ETDRS)
Mean (SD)
BCVA >20/200 (letters read
35)
BCVA
20/200 (letters read
34)
69 (61)
45 (39)
88 (77.2)
5 (4.4)
7 (6.1)
14 (12.3)
95 (83.3)
19 (16.7)
38 (52)
35 (48)
59 (80.8)
5 (6.8)
2 (2.7)
7 (9.6)
64 (87.7)
9 (12.3)
50.7 (13.90)
86 (75.4)
28 (24.6)
48.9 (14.42)
55 (75.3)
18 (24.7)
Retinal ischaemia status, n (%)
Non-ischaemic a
Ischaemic
Indeterminate
77 (67.5)
17 (14.9)
20 (17.5)
50 (68.5)
12 (16.4)
11 (15.1)
Retinal thickness ( μm), mean 661.7 (237.37) 672.4 (245.33)
EDTRS: Early Treatment Diabetic Retinopathy Study; PRN: as-needed; SD: standard deviation.
a Less than 10 disc areas of ischaemia.
Brown DM, et al. Am J Ophthalmol . 2013;155:429-437.
Total (n=187)
66.3 (13.85)
107 (57)
80 (43)
147 (78.6)
10 (5.3)
9 (4.8)
21 (11.2)
159 (85.0)
28 (15.0)
50.0 (14.09)
141 (75.4)
46 (24.6)
127 (67.9)
29 (15.5)
31 (16.6)
15.1 (3.08)
90
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: Baseline characteristics (continued)
Characteristic
IOP (mmHg), mean (SD)
Time since CRVO diagnosis (months)
Mean (SD)
2 months
>2 months
NEI VFQ-25 total score, mean (SD)
NEI VFQ-25 near activities score, mean (SD)
NEI VFQ-25 distance activities score, mean (SD)
Vision dependency score, mean (SD)
Monthly aflibercept aflibercept PRN (n=114)
15.1 (3.26)
2.73 (3.09)
64 (56.1)
49 (43.0)
77.39 (16.176)
69.96 (21.939)
75.99 (21.255)
83.26 (25.511)
Sham aflibercept
PRN (n=73)
15.0 (2.81)
1.88 (2.19)
52 (71.2)
21 (28.8)
77.38 (16.602)
70.72 (20.222)
78.08 (21.258)
82.76 (27.405)
Total (n=187)
15.1 (3.08)
2.40 (2.796)
116 (62.0)
70 (37.4)
77.39 (16.299)
70.25 (21.234)
76.80 (21.224)
83.07 (26.195)
IOP: intraocular pressure; NEI VFQ-25: National Eye Institute Visual Functioning Questionnaire-25; PRN: as-needed;
SD: standard deviation.
Brown DM, et al. Am J Ophthalmol . 2013;155:429-437.
91
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: Study design
Phase 3, randomised, double-masked trial comparing intravitreal aflibercept with sham for macular oedema secondary to CRVO
Treatment-naive patients (N=189) age ≥18 years with macular oedema secondary to CRVO with CRT ≥250 µm and ETDRS BCVA of 20/40 to 20/320
Aflibercept 2 mg monthly (n=115)
Randomisation
3:2
Sham
(n=74)
Treatment to week 24 (N=187) (primary endpoint; proportion of patients gaining
≥15 ETDRS letters in BCVA from baseline to week 24)
Continued active PRN treatment in weeks 24 –52 to all patients for pre-specified endpoints.
End of masked treatment; results reported, sham given if endpoints not reached
Continued treatment in weeks 52 to 100 (PRN extension).
Patients monitored every 12 weeks and received treatment if re-treatment criteria met.
Boyer D, et al. Ophthalmology . 2012;119:1024-1032.
Brown DM, et al. Am J Ophthalmol . 2013;155:429-437.
92
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: study schedule
Week 0 4 8 12 16 20 24 28 32 36 40 44 48 52 64 76 88 100
Monthly aflibercept
Aflibercept PRN
Sham
Aflibercept PRN
Primary
Endpoint
Monthly aflibercept
Sham
Aflibercept PRN
Aflibercept required
Visit w/o injection
93
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS and GALILEO retreatment criteria
• Increase of >50 μm of retinal thickness from lowest previous measurement 1,2
• New/persistent retinal changes or sub-retinal fluid or persistent diffuse oedema ≥ 250 μm in central subfield 1,2
• Loss of ≥5 letters from best previous measurement with any increase in CRT 1,2
• Increase of ≥5 letters between current and most recent visit 1,2
1. Brown DM, et al. Am J Ophthalmol . 2013;155:429-437.
2. Ogura Y et al. Am J Ophthalmology. 2014;158(5)1032-1038.
94
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: Proportion of patients who gained ≥15 letters compared with baseline 1 –3
100%
*P <0.001 vs. Sham
80%
60% 56.1* 55.3*
49.1*
40%
30.1
23.3
20%
12.3
0%
Sham
Week 24
Monthly afliberecept
Week 52
Sham aflibercept PRN
Week 100
Monthly aflibercept aflibercept PRN
Sham n=73; monthly aflibercept n=114. ; LOCF for weeks 52 and 100;
Patients who discontinued before week 24 with fewer than 5 injections were judged as non-responders for week 24 analysis
1. Boyer D, et al. Ophthalmology . 2012;119:1024-1032.
2. Brown DM, et al. Am J Ophthalmol . 2013;155:429-437.
3. Heier JS, et al. Ophthalmology. 2014;121(7):1414-1420.
95
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: Mean change in visual acuity to
24 weeks
20
Mean change in BCVA*
17.3
1†
Aflibercept
Sham
15
10 21.3 letter difference
5
0
0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 80 84 88 92 96 100
Week
†
P <0.001 vs. Sham
-4.0
1
-5
Sham, n 74
Monthly aflibercept, n
115
60 (81.1%)
110 (95.7%)
*Compared to Baseline. LOCF; full analysis set.
1. Boyer D, et al. Ophthalmology . 2012;119:1024-1032.
96
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: Mean change in visual acuity to
52 weeks
20
Mean change in BCVA*
17.3
1†
16.2
2†
Aflibercept
Sham
15
10 21.3 letter difference
All patients switched to aflibercept PRN from week 24
12.4 letter difference
5
3.8
2
0
0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 80 84 88 92 96 100
Week
†
P <0.001 vs. Sham
-4.0
1
-5
Sham, n 74
Monthly aflibercept, n
115
60 (81.1%)
110 (95.7%)
57 (77.0%)
107 (93.0%)
*Compared to Baseline. LOCF; full analysis set.
1. Boyer D, et al. Ophthalmology . 2012;119:1024-1032.
2. Brown DM, et al. Am J Ophthalmol . 2013;155:429-437.
97
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: Mean change in visual acuity to
100 weeks
20
Mean change in BCVA* Aflibercept
Sham
17.3
1.†
16.2
2,†
Patients monitored every 12 weeks
15
13.0
3
10 21.3 letter difference
All patients switched to aflibercept PRN from week 24
12.4 letter difference 11.5 letter difference
5
3.8
2
0
0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 80 84 88 92 96 100
1.5
3
Week
† P <0.001 vs. Sham
4.0
1
-5
Sham, n 74 60 (81.1%) 57 (77.0%) 50 (67.6%)
Monthly aflibercept, n
115 110 (95.7%) 107 (93.0%) 102 (88.7%)
*Compared to Baseline. Sham patients crossed over to aflibercept at 24 weeks. All patients on PRN treatment from week 24. LOCF; full analysis set
1. Boyer D, et al. Ophthalmology . 2012;119:1024-1032.
98
2. Brown DM, et al. Am J Ophthalmol . 2013;155:429-437. 3. Heier JS, et al. Ophthalmology. 2014;121(7):1414-1420.
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: Efficacy by perfusion status
25
20
15
10
5
-10
-15
0
0
-5
8 16 24
32 40 48
Weeks
56 64 72 80
Sham/aflibercept, perfused, n=50
Monthly aflibercept, then aflibercept PRN, perfused n=77
Sham, aflibercept PRN, non-perfused, n=23
Monthly aflibercept, then aflibercept PRN non-perfused, n=37
* perfused: fewer than 10 disc areas of non-perfusion
↓
Patients crossed over from monthly aflibercept to aflibercept PRN or from sham to aflibercept PRN; last observation carried forward (LOCF); full analysis set. ETDRS Early Treatment Diabetic Retinopathy
Study
1. Bayer Healthcare Data on File EYLC003.
88 96
Sham: 5.2 vs. 5.4 injections
Aflibercept: 5.8 vs. 5.2 injections
99
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: Mean change in central retinal thickness to 24 weeks*
Week
0
0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 80 84 88 92 96 100
-100
-200
-300
-144.8
1
Sham
-400
-500
-457.2
1, *
Monthly aflibercept
1. Boyer D, et al. Ophthalmology . 2012;119:1024-1032.
*P <0.001 vs. Sham
* Compared with baseline
LOCF; full analysis set
100
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: Mean change in central retinal thickness to 52 weeks*
Week
0
0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 80 84 88 92 96 100
All patients switched to aflibercept PRN from week 24
-100
-144.8
1
-200
-300
-400
-457.2
1 *
-500
1. Boyer D, et al. Ophthalmology . 2012;119:1024-1032.
2. Brown DM, et al. Am J Ophthalmol . 2013;155:429-437.
-381.8
2
Sham aflibercept PRN
413.0
Monthly aflibercept PRN
2
*P <0.001 vs. Sham
* Compared with baseline
LOCF; full analysis set
101
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: Mean change in central retinal thickness to 100 weeks 1-3 *
Week
0
0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76 80 84 88 92 96 100
All patients switched to aflibercept PRN from week 24
Patients monitored every 12 weeks
-100
-144.8
1
-200
-300
-400
-457.2
1, *
-500
1. Boyer D, et al. Ophthalmology . 2012;119:1024-1032.
2. Brown DM, et al. Am J Ophthalmol . 2013;155:429-437.
3. Heier JS, et al. Ophthalmology. 2014;121(7):1414-1420.
-343.3
3
Sham aflibercept PRN
-381.8
2
413.0
2
*P <0.001 vs. Sham
-390.0
3
Monthly aflibercept
PRN
*Compared with baseline
LOCF; full analysis set
102
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: Aflibercept monthly + aflibercept
PRN patients required fewer injections
40
35
30
25
20
Mean number of injections (weeks 24 –52)
34,6
33,6
33,3
Monthly then PRN aflibercept
25 Sham then PRN aflibercept
20
18,2
16,7
15
10
7,3
6,3
5
5
0
0 1 - 2 3 - 4
Number of injections
5 - 6 7 - 8
Exposure to aflibercept (excluding sham) from weeks 24 to 52 for the week-24 completers within safety analysis set.
Brown DM, et al. Am J Ophthalmol . 2013;155:429-437.
103
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: total PRN injections (weeks 24 –52)
Mean (SD) Min – Max
Sham aflibercept
PRN
(n = 60)
Monthly aflibercept
aflibercept PRN
(n = 110)
3.9 (2.0)
2.7 (1.7)
0 - 8
0 - 8
Median
4
Median time to first PRN injection 1
29 days
3 68 days
1. Brown DM, et al. Am J Ophthalmol . 2013;155:429-437.
104
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS : p roportion of patients with dry retina
Week 24 Week 52 Week 100
100
80 75%
60
40
20
0
15%
54%
57%
34% 34%
Sham
Monthly aflibercept
Sham/
PRN aflibercept
Active/
PRN aflibercept
Sham/
PRN aflibercept
Active/
PRN
Dry retina = absence of any fluid as assessed by OCT.
Active = intravitreal aflibercept 2 mg every 4 weeks.
PRN = intravitreal aflibercept 2 mg as needed from week 24 onwards.
Heier JS, et al. Ophthalmology. 2014;121(7):1414-1420.
.
105
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: Ocular adverse events similar between treatment groups at week 52
Ocular adverse events *
Reduced visual acuity
Conjunctival haemorrhage
Eye pain
Increased intraocular pressure
Monthly aflibercept
aflibercept PRN
Sham aflibercept
PRN
18.4%
16.7%
15.8%
12.3%
21.6%
18.9%
9.5%
13.5%
* Proportion of patients with ≥1 ocular treatment-emergent adverse events; for this study, all adverse events were regarded as 'treatment emergent,' i.e. not seen before treatment or, if already present before treatment, worsened after start of treatment).
Brown DM, et al. Am J Ophthalmol . 2013;155:429-437.
106
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: All ocular serious adverse events from baseline to weeks 24 and 52
Serious adverse events
Weeks 0−24 monthly aflibercept
(n=114)
Weeks 0−24
Sham
(n=74)
Weeks 24−52 monthly aflibercept
aflibercept PRN
(n=110)
Number of patients with
≥1 TEAE in study eye, n (%)
Eye disorders
Vitreous haemorrhage
Glaucoma
Iris neovascularisation
Retinal haemorrhage
Visual acuity reduced
Retinal artery occlusion
Retinal tear
Retinal vein occlusion
Cataract
Cystoid macular oedema
Infections and infestations
Endophthalmitis
Injury, poisoning and procedural complications
Corneal abrasion
4 (3.5%)
2 (1.8%)
0
0
0
0
1 (0.9%)
1 (0.9%)
0
0
0
0
1 (0.9%)
1 (0.9%)
1 (0.9%)
1 (0.9%)
10 (13.5%)
10 (13.5%)
4 (5.4%)
2 (2.7%)
2 (2.7%)
2 (2.7%)
1 (1.4%)
0
1 (1.4%)
1 (1.4%)
0
0
0
0
0
0
3 (2.7%)
3 (2.7%)
1 (0.9%)
0
0
0
0
0
0
1 (0.9%)
1 (0.9%)
1 (0.9%)
0
0
0
0
Brown DM, et al. Am J Ophthalmol . 2013;155:429-437. TEAE: treatment emergent adverse event.
Weeks 24−52 sham
Aflibercept PRN
(n=60)
2 (3.3%)
2 (3.3%)
1 (1.7%)
1 (1.7%)
0
0
0
0
1 (1.7%)
0
1 (1.7%)
0
0
0
0
0 107
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: Patients with study eye ocular SAEs through week 100
Serious adverse events
Number of patients with
≥1 SAE, n (%)
Cataract
Retinal haemorrhage
Visual acuity reduced
Vitreous haemorrhage
Cystoid macular oedema
Macular oedema
Glaucoma
Iris neovascularisation
Retinal tear
Retinal vein occlusion
Retinal artery occlusion
Retinal vascular disorder
Endophthalmitis
Corneal abrasion
Sham
Baseline − Wk 24
(n=74)
10 (13.5%)
Sham aflibercept
Sham aflibercept PRN
Wk 24 – 100
(n=60)
2 (3.3%)
Monthly aflibercept
Baseline − Wk 24
(n=114)
4 (3.5%)
Monthly aflibercept
aflibercept PRN
Wk 24 – 100
(n=110)
8 (7.3%)
0
2 (2.7%)
1 (1.4%)
4 (5.4%)
0
0
2 (2.7%)
2 (2.7%)
1 (1.4%)
1 (1.4%)
0
0
0
0
1. Bayer Healthcare Data on File EYLC001.
1 (1.7%)
0
0
1 (1.7%)
0
0
1 (1.7%)
0
1 (1.7%)
0
0
0
0
0
0
0
1 (0.9%)
0
0
0
0
0
0
0
1 (0.9%)
0
1 (0.9%)
1 (0.9%)
4 (3.6%)
0
1 (0.9%)
1 (0.9%)
2 (1.8%)
1 (0.9%)
0
0
0
1 (0.9%)
0
1 (0.9%)
0
0
108
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: proportion of patients with APTC events
Total deaths (%)
APTC events (%)
Vascular deaths (%)
MI
Arrhythmia
Non-fatal MI
Sham
Baseline − Wk 24
(n=74)
Sham aflibercept PRN
Week 24-52
(n=60)
2 (2.7)
2 (2.7)
2 (2.7%)
1
1
0
0
0
0
0
0
0
Monthly aflibercept
Baseline to week
24
(n=114)
0
0
0
0
0
0
Monthly aflibercept aflibercept PRN
Wk 24 –52
(n=110)
0
1 (0.5)
0
0
0
1 (0.5)
APTC: Anti-platelet Trialists’ Collaboration; MI: myocardial infarction.
1. Bayer Healthcare Data on File EYLC003.
109
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: Conclusions
• Monthly aflibercept resulted in a 21-letter improvement in visual acuity at week 24, compared to sham ( P =0.001) 1
• 24 week treatment delay in sham group resulted in worse visual outcomes vs . aflibercept at 52 weeks (p<0.001) and 100 weeks 1,2 *
• In patients with ischaemic disease: 1
– 51.4% of aflibercept vs. 4.3% sham eyes gained ≥15 letters at week 24
– 48.6% of aflibercept vs. 30.4% sham eyes gained ≥15 letters at week 52
• Like other anti-VEGF studies, visual acuity/anatomic improvements at end of fixed-dosing period reduced with
PRN dosing 2
• Intravitreal aflibercept injection was well tolerated with no new safety signals compared with previous anti-VEGF studies 1,2
1. Brown DM, et al. Am J Ophthalmol . 2013;155:429-437.
2. Heier JS, et al. Ophthalmology. 2014;121(7):1414-1420.
* Statistical difference not tested
110
Date of Prep March 2015
L.GB.01.2014.4924b
COPERNICUS: Summary and key messages
Summary
Mean change in BCVA (letters)
% patients ≥15 letter gain
16.2 at 52 weeks
55.3% at 52 weeks
Mean number of injections 8.7 over 52 weeks (12 over 100 weeks)
Mean change in retinal thickness
(central retinal thickness)
-413 μm at 52 weeks
Key messages
• BCVA gains and reduction in retinal thickness continue to 52 and 100 weeks (but diminished with PRN regimen)
• Immediate therapy gives more BCVA benefit than the six-month delay of sham arm
• ‘Treat and extend’ regimen may be chosen in real-world clinical practice
• No cases of iris neovascularisation in aflibercept-treated patients (1/170 patients treated with monthly and/or PRN aflibercept reported glaucoma)
• Ischaemic patients included in study
Brown DM, et al. Am J Ophthalmol 2013;155:429-437.
111
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Aims and inclusion/exclusion criteria
Aim
To evaluate intravitreal aflibercept for patients with macular oedema secondary to central retinal vein occlusion
Inclusion
Centre involved macular oedema secondary to
CRVO diagnosed ≤9 months before study initiation
Retinal thickness (central subfield) ≥250 μm on OCT
Exclusion
Previous treatment with antiangiogenic drugs, panretinal or macular laser photocoagulation
Uncontrolled glaucoma (IOP ≥ 25 mmHg), filtration surgery
Recent use of intraocular/periocular steroids
Iris neovascularisation
Holz FG, et al. Br J Ophthalmology . 2013;97:278-284.
112
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Baseline characteristics
Characteristic
Age (years)
Mean (SD) (range)
Sex
Male
Female
Race
White
Asian
Not reported
Geographic region, n (%)
Europe
Asia/Pacific
Renal impairment
Normal
Mild
Moderate
Severe
Missing
Hepatic impairment
Yes
No
Retinal ischaemic status
Non-ischaemic
Ischaemic
Indeterminable
Holz FG, et al. Br J Ophthalmology . 2013;97:278-284.
Monthly aflibercept
aflibercept PRN
(n=103)
Sham aflibercept
PRN (n=68)
59.9 (12.4)
58 (56.3)
45 (43.7)
63.8 (13.3)
37 (54.4)
31 (45.6)
74 (71.8)
26 (25.2)
3 (2.9)
73 (70.9)
30 (29.1)
61 (59.2)
36 (35.0)
5 (4.9)
0
1 (1.0)
3 (2.9)
100 (97.1)
89 (86.4)
7 (6.8)
7 (6.8)
49 (72.1)
15 (22.1)
4 (5.9)
48 (70.6)
20 (29.4)
37 (54.4)
17 (25.0)
9 (13.2)
2 (2.9)
3 (4.4)
2 (2.9)
66 (97.1)
54 (79.4)
7 (10.3)
7 (10.3)
Total (n=171)
61.5 (12.9)
95 (55.6)
76 (44.4)
123 (71.9)
41 (24.0)
7 (4.1)
121 (70.8)
50 (29.2)
98 (57.3)
53 (31.0)
14 (8.2)
2 (1.2)
4 (2.3)
5 (2.9)
166 (97.1)
143 (83.6)
14 (8.2)
14 (8.2) 113
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Baseline characteristics (continued)
Characteristic
Time since CRVO diagnosis
<2 months
2 months
Missing
Mean time since CRVO diagnosis in days (SD)
Mean ETDRS BCVA letter score (SD)
ETDRS BCVA >20/200
Mean CRT ( μm) (SD)
Mean IOP (mmHg) (SD)
Monthly aflibercept aflibercept PRN
(n=103)
Sham aflibercept PRN
(n=68)
55 (53.4)
46 (44.7)
2 (1.9)
78.0 (89.6)
53.6 (15.8)
86 (83.5)
683.2 (234.5)
15.1 (2.8)
35 (51.5)
33 (48.5)
0
87.6 (79.1)
50.9 (15.4)
56 (82.4)
638.7 (224.7)
14.4 (2.7)
Total (n=171)
90 (52.6)
79 (46.2)
2 (1.2)
81.8 (85.4)
52.2 (15.7)
142 (83.0)
665.5 (231.0)
14.9 (2.7)
Holz FG, et al. Br J Ophthalmology . 2013;97:278-284.
114
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Study design
Phase 3, randomised, double-masked trial comparing intravitreal aflibercept with sham for macular oedema secondary to CRVO
Treatmentnaive patients (N=177) aged ≥18 years with macular oedema secondary to CRVO with CRT ≥250 µm and ETDRS BCVA of 20/40 to 20/320
Monthly aflibercept
(n=106)
Randomisation
3:2
Sham
(n=71)
Treatment to week 24 (N=152) (primary endpoint; proportion of patients gaining ≥15 letters in BCVA at week 24 compared with baseline) a
Beginning at week 52, both groups received treatment as needed but were monitored every 8 weeks
Continued treatment to week 76 (end of masked treatment) a Beginning at week 24, patients in monthly aflibercept arm dosed as PRN. Patients on sham continue on sham to week 52.
Thereafter, the sham group received aflibercept unless the clinician decided otherwise.
Holz FG, et al. Br J Ophthalmology . 2013;97:278-284.
115
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: study schedule
Week 0 4 8 12 16 20 24 28 32 36 40 44 48 52 60 68 76
Monthly aflibercept
Aflibercept PRN
Sham
Aflibercept PRN
Primary endpoint
Monthly aflibercept
Sham
Aflibercept PRN
Aflibercept required
Visit w/o injection
116
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Proportion of patients who gained ≥15 letters compared with baseline 1 –3
Sham
100
80
60
40
20
22.1
60.2
a
32.4
60.2
b
0
29.4
Week 24 Week 52
Monthly aflibercept
Sham aflibercept PRN
57.3
c
Week 76 a P <0.0001 vs sham.
b P =0.0004 vs sham.
c P <0.001 vs sham.
Monthly aflibercept aflibercept PRN
1. Holz FG et al. Br J Ophthalmol . 2013;97(3):278-284.
2. Korobelnik J-F et al. Ophthalmology . 2014;121(1)202-208.
3. Ogura Y et al.
Am J Ophthalmology.
2014;158(5)1032-1038.
117
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Mean change from baseline in BCVA to
24 weeks
20
+18.0
1 *
Monthly aflibercept
15
10
14.7 letter difference
-5
5
+3.3
1
Sham
0
0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76
Week
*P <0.0001 vs. sham LOCF; full analysis set.
1. Holz FG, et al. Br J Ophthalmology . 2013;97:278-284.
118
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Mean change from baseline in BCVA to
52 weeks
20
15
10
+18.0
1 *
+ 16.9
2 *
14.7 letter difference
Patients crossed over from monthly aflibercept to aflibercept PRN
13.1 letter difference
5
+3.8
2
+3.3
1 Sham patients remained on sham
0
0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76
Week
-5 *P <0.0001 vs. sham
1. Holz FG, et al. Br J Ophthalmology . 2013;97:278-284.
2. Korobelnik J-F et al. Ophthalmology . 2014;121(1)202-208.
LOCF; full analysis set.
119
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Mean change from baseline in BCVA to
76 weeks
20
15
10
+18.0
1 *
+ 16.9
2 *
Monthly aflibercept aflibercept PRN
+13.7
3
14.7 letter difference
Patients crossed over from monthly aflibercept to aflibercept PRN
13.1 letter difference
7.5 letter difference
5
+3.8
2
+6.2
3
Sham aflibercept PRN
0
0 4
+3.3
1 Sham patients remained on sham
Week
Patients crossed over from sham to aflibercept PRN
8-weekly monitoring
†
8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76
†
Sham patients received an aflibercept injection at week 52 unless the clinician decided otherwise
-5
*P <0.0001 vs. sham
1. Holz FG, et al. Br J Ophthalmology . 2013;97:278-284.
2. Korobelnik J-F et al. Ophthalmology . 2014;121(1)202-208.
3. Ogura Y et al. Am J Ophthalmology. 2014;158(5)1032-1038.
LOCF; full analysis set.
120
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Efficacy by perfusion status
25
20
15
10
5
0
0
-5
Weeks
-10
-15
Sham/aflibercept, perfused, n=54
Monthly aflibercept, then aflibercept PRN, perfused n=89
Sham, aflibercept PRN, non-perfused, n=14
Monthly aflibercept, then aflibercept PRN non-perfused, n=14
* perfused: fewer than 10 disc areas of non-perfusion
Sham: 1.5 vs. 2.4 injections
Aflibercept: 3.8 vs. 3.3 injections
↓ Patients crossed over from monthly aflibercept to aflibercept PRN or from sham to aflibercept PRN; last observation carried forward (LOCF); full analysis set. ETDRS Early Treatment Diabetic Retinopathy Study
1. Bayer Healthcare Data on File EYLC003. 2. Korobelnik J-F et al. Ophthalmology . 2014;121(1)202-208.
121
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Mean change in central retinal thickness (CRT) to week 24
-100
-200
-300
-400
-500
Week
0
0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76
Sham
-169.3
1
Monthly aflibercept
-448.6
1 *
*P <0.0001 vs. sham
LOCF; full analysis set.
1. Holz FG, et al. Br J Ophthalmology . 2013;97:278-284.
122
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Mean change in central retinal thickness (CRT) to week 52
Week
0
0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76
-100
Sham patients remain on sham
-200
-300
-169.3
1
Monthly aflibercept patients crossed over to aflibercept
PRN
-219.3
2
-400
-448.6
1 * -423.5
2 *
-500
*P <0.0001 vs. sham
LOCF; full analysis set.
1. Holz FG, et al. Br J Ophthalmology . 2013;97:278-284.
2. Korobelnik J-F et al. Ophthalmology . 2014;121(1)202-208.
123
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Mean change in central retinal thickness (CRT) to week 76
Week
0
0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 64 68 72 76
-100
Sham patients remain on sham
Sham patients crossed over to aflibercept PRN
†
†
Monitoring every
8 weeks
-200 -169.3
1
-300
-219.3
2
Sham aflibercept PRN
–306.4
3
Monthly aflibercept patients crossed over to aflibercept PRN
-400
–389.4
3
Monthly aflibercept aflibercept PRN
-448.6
1 * -423.5
2 *
-500
*P <0.0001 vs. sham
LOCF; full analysis set.
1. Holz FG, et al. Br J Ophthalmology . 2013;97(3):278-284.
2. Korobelnik J-F et al. Ophthalmology . 2014;121(1)202-208.
3. Ogura Y et al. Am J Ophthalmology. 2014;158(5)1032-1038.
124
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: total PRN injections (weeks 24 –52)
Mean (SD) Min – Max
Monthly aflibercept
aflibercept PRN
(n = 97)
2.5 (1.7) 0 - 6
Median
3
Median time to first PRN injection 1
83 days
1. Korobelnik J-F et al. Ophthalmology . 2014;121(1)202-208.
125
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: p roportion of patients with dry retina
Week 24 Week 52 Week 76
100
80
80%
67%
60%
60
40
20
26%
30%
52%
0
Sham Monthly aflibercept
Sham
Monthly aflibercept then aflibercept
PRN
Sham then aflibercept
PRN
Monthly aflibercept then aflibercept
PRN
Dry retina = absence of any fluid as assessed by OCT.
PRN = intravitreal aflibercept 2 mg as needed.
Ogura Y et al. Am J Ophthalmology. 2014;158(5)1032-1038 .
126
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Most aflibercept ocular adverse events associated with injection procedure at week 24
Eye pain
Retinal vascular disorder
Conjunctival haemorrhage
Retinal exudates
Foreign body sensation
Ocular safety
Monthly aflibercept n=104 (%)
12 (11.5)
6 (5.8)
9 (8.7)
7 (6.7)
6 (5.8)
Sham n=68 (%)
3 (4.4)
6 (8.8)
3 (4.4)
5 (7.4)
5 (7.4)
Holz FG, et al. Br J Ophthalmology . 2013;97:278-284.
127
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Other ocular treatment-emergent adverse events (
3% incidence) at week 24
Monthly aflibercept n=104, n (%)
Ocular hyperaemia
Vitreous floaters
Macular oedema
Macular ischaemia
Optic disc vascular disorder
Eye irritation
Lacrimation increased
Papilloedema
Retinal ischaemia
Visual acuity reduced
2 (1.9)
1 (1.0)
0
IOP increased 10 (9.6)
General disorder and administrative site conditions
5 (4.8) Injection site pain
Non-ocular events
Nasopharyngitis
Headache
Hypertension
8 (7.7)
7 (6.7)
4 (3.8)
3 (2.9) Back pain
Arthralgia
Fall
1 (1.0)
0
5 (4.8)
5 (4.8)
4 (3.8)
4 (3.8)
4 (3.8)
3 (2.9)
3 (2.9)
Holz FG, et al. Br J Ophthalmology . 2013;97:278-284.
Sham n=68, n (%)
4 (5.9)
0
11 (16.2)
3 (4.4)
3 (4.4)
7 (10.3)
4 (5.9)
3 (4.4)
3 (4.4)
7 (10.3)
4 (5.9)
2 (2.9)
6 (8.8)
4 (5.9)
3 (4.4)
3 (4.4)
5 (7.4)
3 (4.4)
128
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Patients with serious adverse events in the study eye at weeks 24 –52
Safety analysis set
Number of patients (%) with
≥1 such adverse event
Glaucoma
Iris neovascularisation
Macular oedema
Reduced visual acuity
Vitreous detachment
Vitreous haemorrhage
Macular fibrosis
Macular ischaemia
Retinal detachment
Retinal vein occlusion
Sham + aflibercept
Sham
PRN
(n=57)
(n=68)
2 (3.5%)
1 (1.8%)
0
0
0
0
1 (1.8%)
0
0
0
0
Korobelnik J-F et al. Ophthalmology . 2014;121(1)202-208.
Monthly aflibercept aflibercept PRN
(n=97)
8 (8.2%)
0
0
4 (4.1%)
1 (1.0%)
0
1 (1.0%)
1 (1.0%)
1 (1.0%)
1 (1.0%)
1 (1.0%)
129
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Patients with serious adverse events in the study eye at week 76
Safety analysis set
Number of patients (%) with
≥1 such adverse event
Blindness unilateral
Glaucoma
Iris neovascularisation
Macular fibrosis
Macular ischaemia
Macular oedema
Retinal vein occlusion
Visual acuity reduced
Vitreous detachment
Vitreous haemorrhage
Ogura Y et al. Am J Ophthalmology. 2014;158(5)1032-1038.
6 (8.8%)
0
2 (2.9%)
0
0
0
2 (2.9%)
0
1 (1.5%)
0
1 (1.5%)
Monthly aflibercept aflibercept PRN
(n=104)
11 (10.6%)
1 (1.0%)
0
1 (1.0%)
1 (1.0%)
1 (1.0%)
4 (3.8%)
1 (1.0%)
2 (1.9%)
1 (1.0%)
1 (1.0%)
130
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: proportion of patients with APTC events at week 52
Total deaths (%)
APTC events (%)
Sham
(n=68)
0
0
Monthly aflibercept
aflibercept PRN
(n=104)
0
0
APTC: Anti-platelet Trialists’ Collaboration; MI: myocardial infarction.
Ogura Y et al. Am J Ophthalmology. 2014;158(5)1032-1038.
131
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Aflibercept maintained significantly greater letter gains at week 76
Patients gaining
≥15 ETDRS letters
Mean letter gain
Mean change CRT
Overall results
Monthly aflibercept
aflibercept
PRN
Sham aflibercept
PRN
57.3% 29.4%
13.7
-389.4
μm
6.2
-306.4
μm
P value
<0.001
<0.01
p=0.1122
Ogura Y et al. Am J Ophthalmology. 2014;158(5)1032-1038.
132
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Conclusions
• Monthly aflibercept resulted in rapid, sustained and statistically significant improvement in visual acuity at week 24, compared with sham 1
• 6.8% of aflibercept patients and 10.3% of sham patients had definite ischaemic retinal occlusion 1
• There was a marked improvement in BCVA with aflibercept in the subgroup of patients with nonperfused retinas at baseline, versus a particularly poor response in the nonperfused sham group 2
• Gains in visual acuity benefits were largely maintained during weeks 24 to 52 2,3
• Visual acuity gains were reduced with PRN dosing and infrequent monitoring during weeks 52 to 76 2,3
• Approximate 4 letter loss from week 24 to 76 2,3
• Regimen reflective of real-world clinical practice 2,3
1. Holz FG, et al. Br J Ophthalmology . 2013;97:278-284.
2. Korobelnik J-F et al. Ophthalmology . 2014;121(1)202-208.
3. Ogura Y et al. Am J Ophthalmology. 2014;158(5)1032-1038.
133
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Conclusions
• CRT and percentage of patients without retinal fluid deteriorated when dosing was switched from fixed with monthly monitoring to PRN dosing with infrequent monitoring 1,2
• In the control group, gains in visual acuity with treatment were less pronounced as a result of treatment delay 1,2
• These results indicate potential added benefit with earlier treatment 1,2
• The number of serious adverse events over the 76 weeks were small and balanced between both groups 1-3
1. Holz FG, et al. Br J Ophthalmology . 2013;97:278-284.
2. Korobelnik J-F et al. Ophthalmology . 2014;121(1)202-208.
3. Ogura Y et al. Am J Ophthalmology. 2014;158(5)1032-1038.
134
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO: Summary and key messages
Summary
Mean change in BCVA (letters)
% patients ≥15 letter gain*
16.9 at 52 weeks, 13.7 at 76 weeks 1,2
60.2% at 52 weeks, 57.3% at 76 weeks 1,2
Mean 11.8 over 52 weeks 1 Number of injections
Mean change in retinal thickness (central retinal thickness)
-423
μm at 52 weeks, -389 at 76 weeks 1,2
Key messages
• Rapid and sustained BCVA gains, and reduction in retinal thickness continue to
52 weeks (but diminished with PRN regimen 52
–76 weeks) 1,2
• 1-year delay in treatment for control group resulted in reduced gains in visual acuity 1,2
• Includes ischaemic patients (10.3% in sham arm, 6.8% in aflibercept arm) 1
* Primary endpoint
1. Korobelnik J-F et al. Ophthalmology . 2014;121(1)202-208.
2. Ogura Y et al. Am J Ophthalmology. 2014;158(5)1032-1038.
135
Date of Prep March 2015
L.GB.01.2014.4924b
GALILEO and COPERNICUS: How they compare
GALILEO 1,3
63 centres in Europe and Asia-Pacific
COPERNICUS 2,4
70 centres in US, Canada, Columbia, India and Israel Setting
Design
Primary endpoint
Key secondary endpoints
Randomised, double-masked, 76 weeks,
6 x 2 mg aflibercept or sham every
4 weeks. Protocol driven PRN aflibercept in weeks 24 –52 in aflibercept arm patients only. 8-weekly monitoring and PRN aflibercept available to all patients
52 –76 weeks
Proportion of patients with ≥15 letters
BCVA gain at wk 24 vs. baseline (B)
Aflibercept 60.2%
Sham 22.1% P <0.0001
Change in BCVA from baseline at wk 24 aflibercept 18 letters; sham 3.3 letters
Change in CRT from baseline at week 24 aflibercept -448.6 μm; sham -169.3 μm
Randomised, double-masked, 100 weeks (at request of FDA). 6 x 2 mg aflibercept or sham every 4 weeks in first 24 weeks. Monthly monitoring and protocoldriven PRN aflibercept in weeks 24 –52 in all patients.
12-weekly monitoring and PRN aflibercept in 52 –100 week extension
Proportion of patients gaining ≥15 letters BCVA at wk
24 vs. baseline
Aflibercept 56.1%
Sham 12.3% P <0.001
Change in BCVA from baseline at wk 24 aflibercept 17.3 letters; sham -4.0 letters
Change in CRT from baseline at week 24 aflibercept -457.2 μm; sham -144.8 μm
52 weeks (at request of health authorities) 24 weeks (investigator-driven) Sham treatment
1. Holz FG, et al. Br J Ophthalmology . 2013;97:278-284.
2. Brown DM, et al. Am J Ophthalmol . 2013;155:429-437.
3. Korobelnik J-F et al. Ophthalmology . 2014;121(1)202-208.
4. Boyer D, et al. Ophthalmology . 2012;119:1024-1032.
136
Date of Prep March 2015
L.GB.01.2014.4924b
Image Library – CRVO
CRVO
• Colour fundus showing tortuous retinal veins
Image courtesy of Mrs Deepali Varma, Sunderland Eye Infirmary.
138
Date of Prep March 2015
L.GB.01.2014.4924b
Ischaemic CRVO
• Extensive deep dark haemorrhages
Image courtesy of Mrs Deepali Varma, Sunderland Eye Infirmary.
139
Date of Prep March 2015
L.GB.01.2014.4924b
Non-ischaemic CRVO
Image courtesy of Mrs Deepali Varma, Sunderland Eye Infirmary.
140
Date of Prep March 2015
L.GB.01.2014.4924b
Swollen disc in ischaemic CRVO
• Colour fundus • Fundus fluorescein angiogram
Images courtesy of Mrs Deepali Varma, Sunderland Eye Infirmary.
141
Date of Prep March 2015
L.GB.01.2014.4924b
Non-ischaemic CRVO: Right posterior pole
• Multiple haemorrhages in all
4 quadrants
• Tortuous veins
• Absence of cotton wool spots suggests well-perfused nonischaemic CRVO
Image courtesy of Mr Simon P Kelly, Bolton, UK.
142
Date of Prep March 2015
L.GB.01.2014.4924b
Non-ischaemic CRVO: Right disc
• Swollen right optic disc
• Engorged tortuous veins
• Retinal haemorrhages in all 4 quadrants
Image courtesy of Mr Simon P Kelly, Bolton, UK.
143
Date of Prep March 2015
L.GB.01.2014.4924b
Non-ischaemic CRVO: Zoom of right disc
• Swollen right optic disc
• Blurred disc margins and engorged tortuous veins
• Retinal haemorrhages in all 4 quadrants
Image courtesy of Mr Simon P Kelly, Bolton, UK.
144
Date of Prep March 2015
L.GB.01.2014.4924b
Non-ischaemic CRVO: Left fundus
• Normal calibre retinal veins
• Incidental myelinated nerve fibre inferior
Image courtesy of Mr Simon P Kelly, Bolton, UK.
145
Date of Prep March 2015
L.GB.01.2014.4924b
Right red-free posterior pole
• Haemorrhages seen as black on red-free image
Image courtesy of Mr Simon P Kelly, Bolton, UK.
146
Date of Prep March 2015
L.GB.01.2014.4924b
Right red-free disc
• Haemorrhages seen as black on red-free image
Image courtesy of Mr Simon P Kelly, Bolton, UK.
147
Date of Prep March 2015
L.GB.01.2014.4924b
Right red-free disc close up
• Disc swelling and blurred disc
Image courtesy of Mr Simon P Kelly, Bolton, UK.
148
Date of Prep March 2015
L.GB.01.2014.4924b
Fluorescein angiography, right (50 seconds)
• No area of capillary non-perfusion
• Retinal haemorrhages cause masking
Image courtesy of Mr Simon P Kelly, Bolton, UK.
149
Date of Prep March 2015
L.GB.01.2014.4924b
Fluorescein angiography, right (1 minute)
Image courtesy of Mr Simon P Kelly, Bolton, UK.
150
Date of Prep March 2015
L.GB.01.2014.4924b
FFA right (1.5 minutes)
Image courtesy of Mr Simon P Kelly, Bolton, UK.
151
Date of Prep March 2015
L.GB.01.2014.4924b
Fluorescein angiography, right (1.5 min) zoom
• Close up shows no macular non-perfusion
Image courtesy of Mr Simon P Kelly, Bolton, UK.
152
Date of Prep March 2015
L.GB.01.2014.4924b
Normal fluorescein angiography, left
Image courtesy of Mr Simon P Kelly, Bolton, UK.
153
Date of Prep March 2015
L.GB.01.2014.4924b
Fluorescein angiography, right (5 minutes)
• Leakage at right disc and macular
Image courtesy of Mr Simon P Kelly, Bolton, UK.
154
Date of Prep March 2015
L.GB.01.2014.4924b
Fluorescein angiography, right (5 minutes), zoom
Image courtesy of Mr Simon P Kelly, Bolton, UK.
155
Date of Prep March 2015
L.GB.01.2014.4924b
FFA right (7 minutes)
Image courtesy of Mr Simon P Kelly, Bolton, UK.
156
Date of Prep March 2015
L.GB.01.2014.4924b
Fluorescein angiography, right (7 minutes), zoom
• Late leak of dye at both macula and disc
Image courtesy of Mr Simon P Kelly, Bolton, UK.
157
Date of Prep March 2015
L.GB.01.2014.4924b
Severe central macular oedema: SD-OCT right eye
• Central point macular thickness 591 µm
• Loss of foveal contour with hyporeflective central involving cystic changes
• RPE layer and contour normal
Image courtesy of Mr Simon P Kelly, Bolton, UK.
158
Date of Prep March 2015
L.GB.01.2014.4924b
OCT image of CRVO showing macular oedema
Image courtesy of Mr Ben Burton, Norwich, UK.
159
Date of Prep March 2015
L.GB.01.2014.4924b
Angiogram showing a perfused CRVO
Image courtesy of Mr Ben Burton, Norwich, UK.
160
Date of Prep March 2015
L.GB.01.2014.4924b
Angiogram montage showing a perfused CRVO
Image courtesy of Mr Ben Burton, Norwich, UK.
161
Date of Prep March 2015
L.GB.01.2014.4924b
‘Blood and thunder’ appearance of CRVO on fundoscopy
Image courtesy of Mr Ben Burton, Norwich, UK.
162
Date of Prep March 2015
L.GB.01.2014.4924b
Use of EYLEA (aflibercept)
EYLEA pack contents
164
Date of Prep March 2015
L.GB.01.2014.4924b
Instructions for use
Eylea SmPC.
165
Date of Prep March 2015
L.GB.01.2014.4924b
Method of administration
• Adequate anaesthesia and asepsis, including topical broad-spectrum microbicide applied to the periocular skin, eyelid and ocular surface have to be ensured
• Surgical hand disinfection, sterile gloves, a sterile drape, and a sterile eyelid speculum (or equivalent) are recommended
• The injection needle should be inserted
3.5
–4.0 mm posterior to the limbus into the vitreous cavity, avoiding the horizontal meridian and aiming towards the centre of the globe. The injection volume of 0.05 mL is then delivered; a different scleral site should be used for subsequent injections
• Immediately following the intravitreal injection, patients should be monitored for elevation in intraocular pressure
• Following intravitreal injection patients should be instructed to report any symptoms suggestive of endophthalmitis
Eylea SmPC.
166
Date of Prep March 2015
L.GB.01.2014.4924b
Posology for RVO (branch RVO or central RVO)
• The recommended dose for Eylea is 2 mg aflibercept equivalent to 50 microlitres
• After the initial injection, treatment is given monthly. The interval between two doses should not be shorter than one month
• If visual and anatomic outcomes indicate that the patient is not benefiting from continued treatment, Eylea should be discontinued
• Monthly treatment continues until maximum visual acuity is achieved and/or there are no signs of disease activity. Three or more consecutive, monthly injections may be needed
• Treatment may then be continued with a treat and extend regimen with gradually increased treatment intervals to maintain stable visual and/or anatomic outcomes, however there are insufficient data to conclude on the length of these intervals. If visual and/or anatomic outcomes deteriorate, the treatment interval should be shortened accordingly
• The monitoring and treatment schedule should be determined by the treating physician based on the individual patient’s response
• Monitoring for disease activity may include clinical examination, functional testing or imaging techniques (e.g. optical coherence tomography or fluorescein angiography)
EYLEA SmPC 2015.
167
Date of Prep March 2015
L.GB.01.2014.4924b
Posology: example of a fixed regimen
Monthly dosing until disease is stable
Fixed time between combined monitoring and injection visits
(usually 4 weeks)
Monitor and inject
Monitor and inject
Monitor and inject
4 weeks
Monitor and inject
Stable disease: No change in visual acuity for three consecutive monthly assessments; it might also be necessary to determine anatomic stability
168
Date of Prep March 2015
L.GB.01.2014.4924b
Posology: example of a PRN and treat-to-target regimen
Monthly dosing until disease is stable
Decision whether to inject is taken at monthly monitoring visits
Monitor and inject
?
Monitor
Inject?
?
Monitor
Inject?
4 weeks
?
Monitor
Inject?
Stable disease: No change in visual acuity for three consecutive monthly assessments; it might also be necessary to determine anatomic stability
169
Date of Prep March 2015
L.GB.01.2014.4924b
Posology: example of a treat and extend regimen
Monthly dosing until disease is stable
Loading phase
Time between combined monitoring/injection visits is determined by visual and anatomic outcomes
Maintenance phase
?
Monitor and inject
Monitor and inject?
Extend treatment interval
Extend treatment interval
Monitor and inject
Extend treatment interval
Monitor and inject
Monitor and inject
Stable disease: No change in visual acuity for three consecutive monthly assessments; it might also be necessary to determine anatomic stability
170
Date of Prep March 2015
L.GB.01.2014.4924b
Prescribing information (1)
Eylea ® 40 mg/ml solution for injection in a vial (aflibercept)
Prescribing Information
(Refer to full Summary of Product Characteristics (SmPC) before prescribing)
Presentation: 1 ml solution for injection contains 40 mg aflibercept. Each vial contains 100 microlitres, equivalent to 4 mg aflibercept.
Indication(s): Treatment of neovascular (wet) age-related macular degeneration (AMD), macular oedema secondary to retinal vein occlusion (branch RVO or central RVO) and visual impairment due to diabetic macular oedema (DMO) in adults.
Posology & method of administration: For intravitreal injection only. Must be administered according to medical standards and applicable guidelines by a qualified physician experienced in administering intravitreal injections. Each vial should only be used for the treatment of a single eye. The vial contains more than the recommended dose of 2 mg. The extractable volume of the vial (100 microlitres) is not to be used in total. The excess volume should be expelled before injecting. Refer to SmPC for full details.
Adults: The recommended dose is 2 mg aflibercept, equivalent to 50 microlitres. For wAMD treatment is initiated with one injection per month for three consecutive doses, followed by one injection every two months. No requirement for monitoring between injections.
After the first 12 months of treatment, treatment interval may be extended based on visual and/or anatomic outcomes. In this case the schedule for monitoring may be more frequent than the schedule of injections. For RVO (branch RVO or central RVO), after the initial injection, treatment is given monthly at intervals not shorter than one month. Discontinue if visual and anatomic outcomes indicate that the patient is not benefiting from continued treatment. Treat monthly until maximum visual acuity and/or no signs of disease activity. Three or more consecutive, monthly injections may be needed. Treatment may then be continued with a treat and extend regimen with gradually increased treatment intervals to maintain stable visual and/or anatomic outcomes, however there are insufficient data to conclude on the length of these intervals. Shorten treatment intervals if visual and/or anatomic outcomes deteriorate.
The monitoring and treatment schedule should be determined by the treating physician based on the individual patient’s response. For DMO, initiate treatment with one injection/month for 5 consecutive doses, followed by one injection every two months.
No requirement for monitoring between injections. After the first 12 months of treatment, the treatment interval may be extended based on visual and/or anatomic outcomes. The schedule for monitoring should be determined by the treating physician.
If visual and anatomic outcomes indicate that the patient is not benefiting from continued treatment, treatment should be discontinued.
Hepatic and/or renal impairment: No specific studies have been conducted. Available data do not suggest a need for a dose adjustment.
Elderly population: No special considerations are needed. Limited experience in those with DMO over 75years old.
Paediatric
population: No data available.
Contra-indications: Hypersensitivity to active substance or any excipient; active or suspected ocular or periocular infection; active severe intraocular inflammation.
Warnings & precautions: As with other intravitreal therapies endophthalmitis has been reported. Aseptic injection technique essential.
Patients should be monitored during the week following the injection to permit early treatment if an infection occurs. Patients must report any symptoms of endophthalmitis without delay. Increases in intraocular pressure have been seen within 60 minutes of intravitreal injection; special precaution is needed in patients with poorly controlled glaucoma (do not inject while the intraocular pressure is
≥ 30 mmHg). Immediately after injection, monitor intraocular pressure and perfusion of optic nerve head and manage appropriately. There is a potential for immunogenicity as with other therapeutic proteins; patients should report any signs or symptoms of intraocular inflammation e.g
pain, photophobia or redness, which may be a clinical sign of hypersensitivity.
Systemic adverse events including non-ocular haemorrhages and arterial thromboembolic events have been reported following intravitreal injection of VEGF inhibitors. Safety and efficacy of concurrent use in both eyes have not been systemically studied. No data is available on concomitant use of Eylea with other anti-
VEGF medicinal products (systemic or ocular). Caution in patients with risk factors for development of retinal pigment epithelial tears including large and/or high pigment epithelial retinal detachment. Withhold treatment in patients with: rhegmatogenous retinal detachment or stage 3 or 4 macular holes; with retinal break and do not resume treatment until the break is adequately repaired. Withhold treatment and do not resume before next scheduled treatment if there is: decrease in best-corrected visual acuity of
≥30 letters compared with the last assessment; central foveal subretinal haemorrhage, or haemorrhage ≥50%, of total lesion area. Do not treat in the 28 days prior to or following performed or planned intraocular surgery. Eylea should not be used in pregnancy unless the potential benefit outweighs the potential risk to the foetus.
Women of childbearing potential have to use effective contraception during treatment and for at least 3 months after the last intravitreal injection. Populations with limited data: There is limited experience of treatment with Eylea in patients with ischaemic, chronic RVO. In patients presenting with clinical signs of irreversible ischaemic visual function loss, aflibercept treatment is not recommended. There is limited experience in
DMO due to type I diabetes or in diabetic patients with an HbA1c over 12% or with proliferative diabetic retinopathy. Eylea has not been studied in patients with active systemic infections, concurrent eye conditions such as retinal detachment or macular hole, or in diabetic patients with uncontrolled hypertension. This lack of information should be considered when treating such patients.
171
Date of Prep March 2015
L.GB.01.2014.4924b
Prescribing information (2)
Eylea ® 40 mg/ml solution for injection in a vial (aflibercept)
Prescribing Information
(Refer to full Summary of Product Characteristics (SmPC) before prescribing)
Interactions: No available data.
Fertility, pregnancy & lactation: Not recommended during pregnancy unless potential benefit outweighs potential risk to the foetus. No data available in pregnant women. Studies in animals have shown embryo-foetal toxicity. Women of childbearing potential have to use effective contraception during treatment and for at least 3 months after the last injection. Not recommended during breastfeeding. Excretion in human milk: unknown. Male and female fertility impairment seen in animal studies with high systemic exposure not expected after ocular administration with very low systemic exposure.
Effects on ability to drive and use machines: Possible temporary visual disturbances. Patients should not drive or use machines if vision inadequate.
Undesirable effects: Very common : conjunctival haemorrhage (phase III studies: increased incidence in patients receiving antithrombotic agents), visual acuity reduced.
Common: retinal pigment epithelial tear, detachment of the retinal pigment epithelium, retinal degeneration, vitreous haemorrhage, cataract (nuclear or subcapsular), corneal abrasion or erosion, corneal oedema, increased intraocular pressure, blurred vision, vitreous floaters, vitreous detachment, injection site pain, eye pain, foreign body sensation in eyes, increased lacrimation, eyelid oedema, injection site haemorrhage, punctate keratitis, conjunctival or ocular hyperaemia.
Uncommon: Injection site irritation, abnormal sensation in eye, eyelid irritation.
Serious: cf. CI/W&P - in addition: blindness, endophthalmitis, cataract traumatic, transient increased intraocular pressure, vitreous detachment, retinal detachment or tear, hypersensitivity (incl. allergic reactions), vitreous haemorrhage, cortical cataract, lenticular opacities, corneal epithelium defect/erosion, vitritis, uveitis, iritis, iridocyclitis, anterior chamber flare. Consult the SmPC in relation to other side effects.
Overdose: Monitor intraocular pressure and treat if required .
Incompatibilities: Do not mix with other medicinal products.
Special Precautions for
Storage: Store in a refrigerator (2 °C to 8°C). Do not freeze. Unopened vials may be kept at room temperature (below 25 °C) for up to 24 hours before use.
Legal Category:
POM.
Package Quantities & Basic NHS Costs: Single vial pack
£816.00.
MA
Number(s): EU/1/12/797/002.
Further information available from: Bayer plc, Bayer
House, Strawberry Hill, Newbury, Berkshire RG14 1JA, United Kingdom. Telephone:
01635 563000.
Date of preparation: March 2015
Eylea
® is a trademark of the Bayer Group
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard . Adverse events should also be reported to Bayer plc. Tel.: 01635 563500,
Fax.: 01635 563703, Email: pvuk@bayer.com
172
Date of Prep March 2015
L.GB.01.2014.4924b