Chapter 2b
advertisement

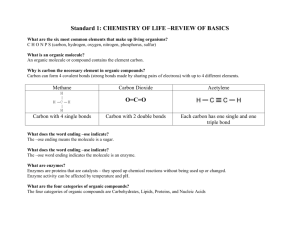
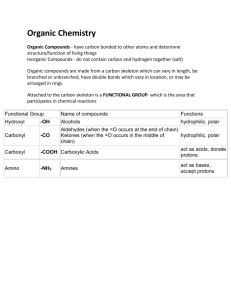
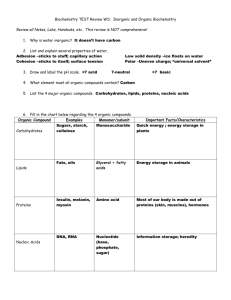
TORTORA • FUNKE • CASE Microbiology AN INTRODUCTION EIGHTH EDITION Chapter 2, part B Chemical Principles Important Biological Molecules • Organic compounds always contain carbon and hydrogen. • Inorganic compounds typically lack carbon. Inorganic Compounds: Water • Polar molecule Figure 2.4a Inorganic Compounds: Water • Solvent – Polar substances dissociate, forming solutes Figure 2.5 Inorganic Compounds: Water • H+ and OH participate in chemical reactions Inorganic Compounds: Water • Hydrogen bonding between water molecules makes water a temperature buffer. Figure 2.4b Acids, Bases, and Salts • An acid is a substance that dissociates into one or more H+. HCl H+ + Cl Figure 2.6a Acids, Bases, and Salts • A base is a substance that dissociates into one or more OH. NaOH Na+ + OH Figure 2.6b Acids, Bases, and Salts • A salt is a substance that dissociates into cations and anions, neither of which is H+ or OH. NaCl Na+ + Cl Figure 2.6c Acid-Base Balance • The amount of H+ in a solution is expressed as pH. • pH = log[H+] • Increasing [H+], increases acidity. • Increasing [OH] increases alkalinity. • Most organisms grow best between pH 6.5 and 8.5. Acid-Base Balance Figure 2.7 • The chain of Organic carbon atoms in an organic molecule is the carbon skeleton. • Functional groups are responsible for most of the chemical properties of a particular organic Compounds Table 2.3.1 Organic Compounds • Small organic molecules can combine into large macromolecules. • Macromolecules are polymers consisting of many small repeating molecules. • The smaller molecules are called monomers. Organic Compounds • Monomers join by dehydration synthesis or condensation reactions. Figure 2.8 Carbohydrates • Are important for structure and as energy sources. • Also for structures like cell walls • Consist of C, H, and O with the formula (CH2O)n Figure 2.8 Carbohydrates • Monosaccharides are simple sugars with 3 to 7 carbon atoms. Figure 2.8 Carbohydrates • Disaccharides are formed when two monosaccharides are joined in a dehydration synthesis. • Disaccharides can be broken down by hydrolysis. Figure 2.8 Carbohydrates • Oligosaccharides consist of 2 to 20 monosaccharides. • Polysaccharides consist of tens or hundreds of monosaccharides joined through dehydration synthesis. • Starch, glycogen, dextran, and cellulose are polymers of glucose that are covalently bonded differently. • Chitin is a polymer of two sugars repeating many times. What is Chitin? Lipids • Three types of lipids: – Simple fats also known as triglycerides – Phospholipids – Steroids • Are the primary components of cell membranes. • Function as storage of energy, membrane structure and some act as hormones (steroids). • Consist of C, H, and O. • Are nonpolar and insoluble in water. Simple lipids • Called fats or triglycerides contain glycerol and fatty acids; formed by dehydration synthesis. • Unsaturated fats have one or more double bonds in the fatty acids. Figure 2.9c Phospholipids lipids • Contain C, H, and O + P, N, or S. • Membranes are made of phospholipids Figure 2.10a Steroids • Consist of four carbon rings, with an –OH group attached to one ring. • Are part of membranes. Figure 2.11 Proteins • Are essential in cell structure and function as enzymes. • Enzymes are proteins that speed chemical reactions. • Transporter proteins move chemicals across membranes. • Flagella are made of proteins. • Some bacterial toxins are proteins. Proteins • Consist of monomer subunits called amino acids. Table 2.4.1 Proteins Table 2.4.2 Amino Acids • Exist in either of two stereoisomer s, D or L. • L-forms are most often found in nature. Figure 2.13 Peptide Bonds • Peptide bonds between amino acids are formed by dehydration synthesis. Figure 2.14 Levels of Protein Structure • The primary structure is a polypeptide chain Figure 2.15a Levels of Protein Structure • The secondary structure occurs when the amino acid chain folds and coils in a regular helix or pleats. Figure 2.15b Levels of Protein Structure • The tertiary structure occurs when the helix folds irregularly, forming disulfide bonds, hydrogen bonds, and ionic bonds between amino acids in the chain. Figure 2.15c Levels of Protein Structure • The quaternary structure consists of two or more polypeptides. Figure 2.15d Level of Protein Structure • Conjugated proteins consist of amino acids and other organic molecules: • Glycoproteins • Nucleoproteins • Lipoproteins Nucleic Acids • Consist of monomer units called nucleotides. • Nucleotides consist of a: • Pentose • Phosphate group • Nitrogen-containing (purine or pyrimidine) base • Adenine, thymine, guanine or cytosine Figure 2.16 DNA • Has the sugar deoxyribose • Exists as a double helix with sugar and phosphate as backbone • A hydrogen bonds with T • C hydrogen bonds with G Figure 2.16 RNA • Has sugar ribose • Is single-stranded • A hydrogen bonds with U • C hydrogen bonds with G Figure 2.17 ATP • Has ribose, adenine, and 3 phosphate groups Figure 2.18 ATP • Is made by dehydration synthesis. • Is broken by hydrolysis to liberate useful energy for the cell.