Single Replacement Reactions DOCX
advertisement

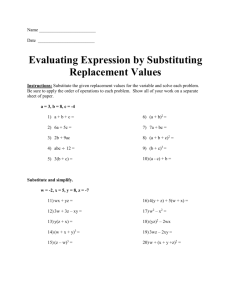
LAB 9 “Single replacement reactions” Introduction: Single replacement reactions are reactions where one element replaces another in a compound. Equations are simple for these reactions, eg. A + BC AC + B where A,B, and C represent different element symbols. Purpose: Determine what single replacement reactions occur, if any, with the materials provided. Tasks: (1) Create a lab report using the standard template provided in word. (2) Using the lab equipment on the bench to investigate the possibility of a single replacement reactions between water and 3 metals, (3)Using the lab equipment on the bench investigate the possibility of a single replacement reactions between HCl (aq) and the 3 metals. (4) Include in the claim the equations (balanced) for reactions discovered. (5) Include in the claim any reactions that do NOT occur. Notes: (1) Make sure to reference the observation data (proof) in the reasoning (conclusion). (2) Do not use the first person in the lab report (cannot use words such as, ”I, we, us, etc.”) (3) Create a table to record your observations, a 4 X 3 table is a good start. There may be as many as 6 reactions, 3 with water and 3 with HCl (aq) (4) Use the equipment in the lab table kits, ONLY use tap water for clean up! (5) All lab procedures MUST be approved by a lab instructor for safety purposes (6) This lab is messy, make sure to clean all the equipment!