enzyme pogil
advertisement
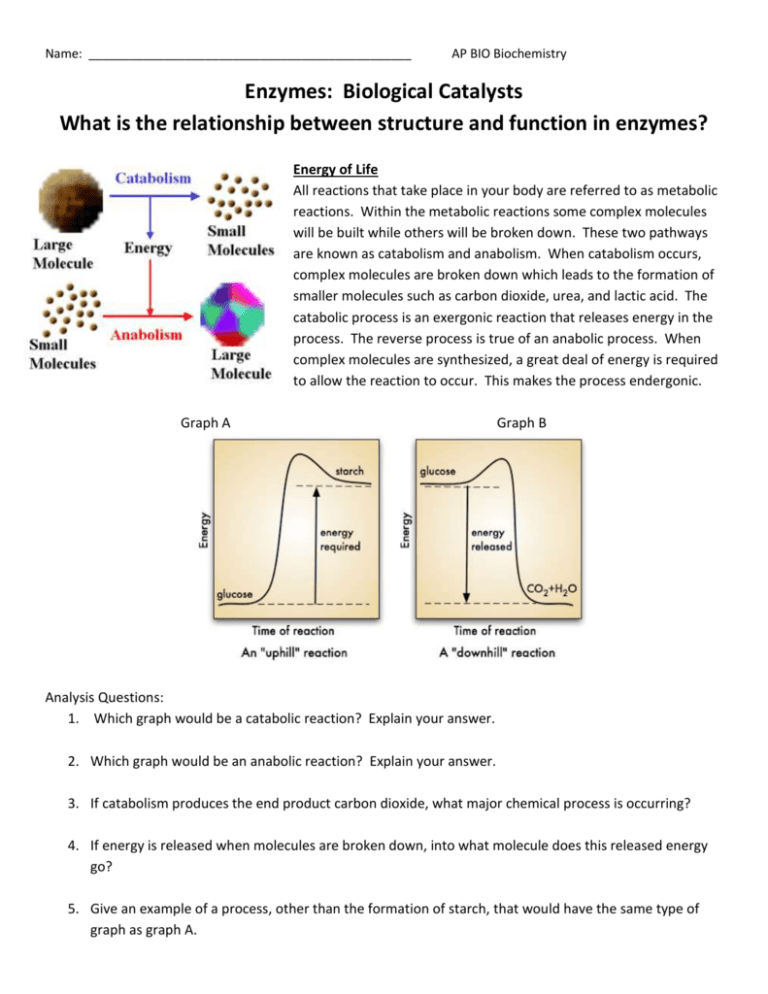
Name: _______________________________________________ AP BIO Biochemistry Enzymes: Biological Catalysts What is the relationship between structure and function in enzymes? Energy of Life All reactions that take place in your body are referred to as metabolic reactions. Within the metabolic reactions some complex molecules will be built while others will be broken down. These two pathways are known as catabolism and anabolism. When catabolism occurs, complex molecules are broken down which leads to the formation of smaller molecules such as carbon dioxide, urea, and lactic acid. The catabolic process is an exergonic reaction that releases energy in the process. The reverse process is true of an anabolic process. When complex molecules are synthesized, a great deal of energy is required to allow the reaction to occur. This makes the process endergonic. Graph A Graph B Analysis Questions: 1. Which graph would be a catabolic reaction? Explain your answer. 2. Which graph would be an anabolic reaction? Explain your answer. 3. If catabolism produces the end product carbon dioxide, what major chemical process is occurring? 4. If energy is released when molecules are broken down, into what molecule does this released energy go? 5. Give an example of a process, other than the formation of starch, that would have the same type of graph as graph A. Movement of Energy - Energy is released and used in catabolic and anabolic reactions. The “middle man” for these two pathways is ATP. 6. Label the two sides of the ATP cycle as anabolism and catabolism. 7. Based on the diagram, in which part of the ATP molecule would stored energy be found? 8. Which side is the result of an exergonic reaction? Label it in the diagram. 9. Which side is the result of an endergonic reaction? Label it in the diagram. Activation Energy Catalysts speed up the rate of a reaction without being altered in the reaction. They work by lowering the energy needed to get the reaction started. Reactions can still occur without enzymes present but the reactants must absorb more energy from their surroundings than reactants in which enzymes are present. This initial energy is called the activation energy. 10. Which label in the graph represents the activation energy without enzymes? _____ 11. Which label in the graph represents the reactants? ______ 12. Which label in the graph represents the total amount of energy gained in this reaction? _______ 13. Which label in the graph represents the activation energy with enzymes? _______ 14. After examining the graph, why do you think the reactions with and without enzymes both end at the same energy level when the products are produced. How enzymes work Enzymes are catalysts found in living organisms. The reactant that the substrate works on is called the substrate. In order for the enzyme to act upon the substrate, the substrate must bind to the active site on the enzyme. This binding of the substrate to the active site forms the enzyme substrate complex. The products are the result of the reaction. Because of the specific shape of the protein, each enzyme will only work on a specific substrate. If an enzyme comes into contact with a substrate that does not fit into the active site there will be no reaction. The active site is not rigid like a key fitting into a lock. Rather the active site will have interactions between the chemical groups on the substrate and those on the side chains of the protein causing a conformational change. This change leads to an induced fit that brings the chemical groups of the active site into position to catalyze the reaction. 15. What differences do you notice comparing the 2 models? 16. In which diagram does the substrate fit exactly? 17. In which diagram would the substrate cause the enzyme’s active site to change shape? _______ Explain why this is a more realistic example than the lock and key model. 18. Are the reactions occurring anabolic or catabolic? ___________ Explain your answer. 19. Once a reaction is complete, enzymes can be used again. Based on the diagrams, explain why the enzymes can be used again. 20. Explain why the substrate in diagram a cannot be acted upon by the enzyme in diagram b? Inhibiting Enzymes An inhibitor is anything that stops an enzyme from working. A competitive inhibitor binds to the same active site as the substrate. If it is removed from the active sire, the enzyme is then free to continue working with a substrate. A non-competitive inhibitor binds somewhere else on the enzyme, which often permanently damages the enzyme. 21. Sometimes the competitive inhibitor can be overcome by increasing the concentration of the substrate molecules. Why do you think this is possible? 22. Non-competitive inhibitors bind to the enzyme but at a location away from the active site. This still prevent s the substrate from binding to the enzyme even though the inhibitor is not directing impacting the active site. Examine the picture and explain why a non-competitive inhibitor prevents substrates from binding. 23. Why is a non-competitive inhibitor more harmful than a competitive inhibitor? 24. Antibiotics, such as penicillin, are effective at killing bacteria. The antibiotic is responsible for blocking the active site of the enzyme in bacteria which forms cell walls. Would penicillin be considered a competitive or non-competitive inhibitor? Explain your answer. Substrate Concentration The rate of enzyme action also changes with the amount of substrate. 25. Based on the graph, explain what happens to the rate of the reaction as the amount of substrate increases. 26. Why do you think the graph does not show an exponential rate for enzyme activity? Other Factors Affecting the Rate of Enzyme Action For a given enzyme, its effectiveness can be measured by how fast it forms products, or by how fast the substrate is used up. When an enzyme is heated it gains energy allowing for more movement and more collisions. Each enzyme has a specific point at which it works the back. Once the temperature of the solution becomes too high the thermal agitation begins to disrupt the bonds that stabilize the enzymes active confirmation, causing the protein to become denatured. This causes a conformational change back to the primary structure of a protein. Enzymes are sensitive to changes in pH. There is usually an optimum pH at which the rate of the reaction in greatest. Different enzymes have different optimal pH values. For example, enzymes in the mouth and small intestine work best in slightly alkaline conditions, but those in the stomach work best in acidic conditions. 27. What happens to the enzyme as the temperature is increased towards the optimal temperature? 28. What is the optimal temperature for the typical human enzyme and what does this represent? 29. What is the optimal temperature for the enzyme found in thermophilic bacteria? 30. Explain why the rate of enzyme action decreases below the optimal temperature but does not denature. 31. When a researcher needs to make extra copies of DNA, they will heat a DNA strand up and use an enzyme to allow the reaction to occur. Explain the conditions they would need to use for human DNA compared to thermophilic bacteria. 32. Above what temperature does the enzyme start to denature in the human? _____________ in the bacteria? ______________ 33. Unlike temperatures for enzymes, when an enzyme is placed into a pH that is higher or lower than the optimum it denatures. Explain why this occurs. 34. When an enzyme denatures, why would it no longer function? Explain in terms of protein structure and folding why this occurs. Allosteric Enzymes An allosteric enzyme is an enzyme in the quaternary structure involved in the regulation of cell processes. These enzymes play a role in cell metabolism by catalyzing various events, like other enzymes, and they can also be used to control the rate of metabolism. These enzymes are used by the cell to keep operating efficiency high and to prevent wastes of energy and unnecessary production inside the cell. Researchers who study allosteric enzymes often work with bacteria such as Escherichia coli to learn more about how these enzymes function and what happens when their structure changes. Function of enzymes can be changed by binding of inhibitors or activators to the active site on the enzyme. With an allosteric enzyme, inhibitors or activators, also known as effectors or regulator molecules, actually bind to a difference site on the enzyme. This changes the structure of the enzyme, consequently altering its function. When an inhibitor binds to an allosteric enzyme, the enzyme is effectively turned off and no longer able to function in the body. Activators, on the other hand, turn the enzyme on so that it can perform a function. The regulator molecules can detach as needed. Using this system, cells can regulate the activity of enzymes in response to changing situations, activating enzymes as needed and deactivating them when it doesn’t want them to work. The binding site where a regulator molecule attaches is known as the allosteric site on the enzyme. Allosteric regulation of enzymes involves a number of different molecules which can fit on this site, much like keys fit into a lock. With inhibition the enzyme is maintained so that it will be ready when needed but won’t function before it is required, and with activation, the allosteric enzyme is kicked into gear so that it can perform a desired metabolic function. The body balances the numbers of active and inactive enzymes to modulate a wide variety of biological processes. 35. Look at the active form of the allosteric enzyme. Notice the active site and the activator site in the stabilized active form of the enzyme and explain why it is referred to as the active form. 36. Explain the inactive form of the enzyme and what this means for chemical reactions in the body. 37. How is the inhibitor in an allosteric enzyme different from a non-competitive inhibitor for an enzyme?




