Postpartum Seizures: A Case Presentation
advertisement

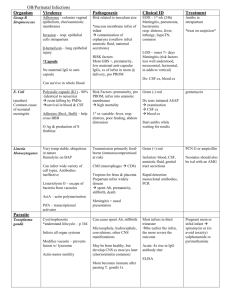
Postpartum HA with Seizures: “to do a blood patch or not to do” Contents: • Presentation of Case Study • Review of CSF infections and findings • Review of differential diagnosis for peripartum seizures • Current literature review • Discussion Case Presentation• A 5’ 7”, 152 #, 17 y.o. at post-partum day 7, • • presented to HR L&D on a Friday afternoon with h/o a GTC seizure earlier that day. She also c/o a bifrontal HA which was worse with sitting/standing, slight stiff neck, cycloplegia, photophobia, mild fever (100 F), No N/V. Pt’s mother stated she observed legs jerking and foaming from the mouth for approx 3-4 min. Pt had loss of urine and post-ictal like state as described by her mother. Recent Obstetrical history • PMHx: “anxiety attacks”. Denied Tob, ETOH, drugs. Took no • • • meds and had no drug allergies. Pt was a G1P1 who 7 days earlier had an uneventful SVD at 39 6/7 wks with a uncomplicated labor epidural for analgesia. During her stay she was not treated for pre-eclampsia, but did have elevated Bp’s in the 140’s/ 90’s and a single increased TP:Cr ratio of 446 (N <150). So- she delivered on a Friday night, was seen in follow-up by anesthesia the next day and seemingly had no issues. D/Ced to home on Sunday in good health And the plot thickens… • Pt was treated in the ED on Tues night with IVF and • • caffeine for a suspected PDPH. She was D/Ced from the ED with PO ibuprofen. Pt was not evaluated by anesthesia during this visit because of easily resolved symptoms with the aforementioned treatment regimen Thus, because of her continued “PDPH” symptoms once she hit the HR floor, the OB Dr.’s wanted us to perform a blood patch. Further Work-up…Would you now do a Blood Patch? • NO ! • One must R/O the possibility of a intracranial hematoma/bleed first and then R/O a CSF infectious process • Recall: Pt had combination of fever, HA, Sz, stiff neck, visual changes; with no N/V. What actions to take next… • In collaboration with the OB attending, it was decided that we • should get a CT of the pt’s head to R/O mass/lesion first---CT was unremarkable. LP was then (attempted multiple times at 3 different levels) performed by the medicine team of doctors. During this time she was also being treated for severe preeclampsia and given Magnesium; – Negative findings for this diagnosis- BP’s were 130’s/70’s, she was not oliguric, Uric acid level WNL, no signs of hemolysis as CBC was WNL, no peripheral edema noted, liver enzymes WNL’s and plts were over 300. – Positive findings included- spot urine protein was 10.9 (0.0-10.0), TP:Cr 330 and she did exhibit Sz, HA, visual disturbances. Pt’s CSF Findings: Cells:100, Polys:23%, Glu:41, Prot:81, WBC:59; gs and cx-, equine enceph-; bld cx -, HIV Ab – Infect. Cells Polys Glucose Protein bacterial meningitis 50010,000/mL > 90% < 40 mg/dL >150 mg/dL Aseptic Meningitis 10-500/mL Early > 50% Normal Late<20% <100 mg/dL Syphilitic meningitis 50-500/mL < 10% <40 mg/dL <100 mg/dL HSV encephalitis 0-1000/mL <50% Normal < 100mg/dL Review of Characteristics of Abnormal CSF : • Bright Red- indicates acute hemorrage • Xanthochromia (yellowish to light red discoloration)• • • breakdown of RBC’s Cloudiness- turbidity indicates infection due to increased WBC’s or protein Elevated Protein- assoc w/ CNS tumors, viral meningitis, hemorrage, MS, GBS Elevated WBC’s– Lymphocytes: viral or TB meningitis, MS, HSV, Syphillis, CNS tumors – Granulocytes: bacterial meningitis Abnormal CSF characteristics cont. • Decreased glucose- bacterial meningitis, SAH • Elevated Lactate- inc glucose metabolism assoc c bacterial or fungal meningitis • CSF pressures- (N in lat recumb position are 60-180 mm H2O) – Increased- space occupying lesions, hydrocephalus, SAH, intracranial infections, severe head injury, and hypoxic/ischemic insults – Decreased- spontaneous intracranial hypotension, colloid cyst of the third ventricle and PDPH. Anesthetic considerations: • Peripartum Seizures- Differential Diagnosis: – Must rule out life threatening medical issues first • • • • • A) Cerebrovascular compromise B) Mass lesions C) infectious diseases D) Metabolic disorders/ epilepsy E) Eclampsia A) Cerebrovascular Compromise • Cerebral infarction • Cerebral hemorrhage • Subarachnoid hemorrhage* • Cerebral venous • • thrombosis* Cerebral edema Malignant HTN B) Mass lesions • A-V malformations* • Benign and/or malignant tumors • Cerebral abcess C) Infectious Diseases • • • • • Viral Bacterial Parasitic infestations HIV Fungal D) Metabolic Disorders/ Epilepsy • • • • • Hyponatremia Hypocalcemia Hypo or Hyperglycemia Central stimulants- cocaine, theophylline etc. Idiopathic epilepsy E) Severe Preeclampsia • In OB, this is obviously one of the most common etiologies Then comes the Neurology Consult… • Impression: Postpartum fever, HA, Seizure= clinical meningoencephalitis involving a viral or aseptic etiology. No focal neurological Sx. – Although one can not exclude early bacterial, listeria or paramenigeal process – Mentioned, but was doubtful about a diagnosis of chemical meningitis secondary to PO ibuprofen or of pleocytosis from low-pressure (CSF <60 mm H2O) or from Sz alone. * Note: the medicine team never got an opening pressure Neurology Consult Cont. • Treatment Plan: - Dilantin, cont. Magnesium for Sz - Empiric Abx Txmnt: acyclovir until HSV studies came back negative; ceftriaxone and vanco given until all bld and CSF cultures came back negative - ID consult, send CSF for viral studies (HSV PCR, enteroviral, arboviral, HIV) - Get MRI of head MRI Results 2 days later: • Findings “consistent with intracranial hypotension.” – Including T1-weighted images with diffuse meningeal gadolinium enhancement with or without subdural and extraarachnoid fluid collections or evidence of descent of the cerebellar tonsils. Furthermore, there is a thick, homogeneous pattern to the extra-axial fluid over the hemispheres bilaterally representing cerebral venous engorgement. – Can also see enlargement of the pituitary gland in cases of intracranial hypotension (Alvarez-Linera et al., Neurology. 2000;55;1895-1897) as the radiologist did appreciate with our pt. MRI Findings: axial T1-WI c gad enhace Case continuation: HD #4 • MRI results back and focus shifts to PDPH • • secondary to CSF leak and intracranial hypotension. Pt seen by anesthesia, HA resolving; not increasingly worse from laying down sittting standing. No indication for blood patch at this time. Pt D/C home on phenytoin and was suppose to f/u with neurologist as outpt. – Of note: pt had no further Sz activity during hospital stay Summary: • 17 y.o. on post-partum day 7 with GTC Sz, HA, visual changes and fever. – Considerations/Proposed questions: • Was it a sequela of Severe Pre-eclampsia? • Could it have been from an unintentional dural puncture when performing the labor epidural? • Was it an aseptic meningitis secondary to a virus or to a chemical? • Who knows? • How common are these outcomes? Let’s look at ways to avoid these clinical symptoms and potential complications in the future. – First, let’s look deeper into the syndrome of intracranial hypotension. Intracranial hypotension: • Pathophysiology behind MRI findings– Monroe-Kellie Doctrine or Rule: states that when the volume of any of the three cranial components (brain parenchyma, CSF, and blood) increases, the volume of one of the others must decrease or the ICP will rise when considering an intact cranial vault. Thus, if CSF volume decreases (ie. a leak), the compensatory mechanism to maintain ICP is by increasing blood volume, especially in the venous capacitance system Intracranial hypotension • A syndrome occurring secondary to various etiologies – Can occur spontaneously; secondary to anything that causes a decrease production of CSF,or increased absorption; or a disruption of a compartment as seen in dural tears. – No matter what the etiology, we treat this condition most commonly with a blood patch or conservatively with IV caffeine, IVF and pain meds Can preeclampsia occur on postpartum day 7 ? • International Journal of Obstetric Anesthesia, Akerman and Hall (from UK), April 2005,14(2), 163166 presented a case of a 29 y.o. in her 3rd pregnancy developed Sz on PPD 7. – No evidence of preeclampsia antepartum or postpartum. Only symptoms preceding sz were fever, HA and visual disturbances. – She c/o sudden onset of frontal and occipital HA on PPD 3 which was worse on standing. Over the course of the next 3 days, HA remained unchanged other than a fluctuation in severity. No focal neurological signs. Cont. – On PPD 7, HA worsened, was febrile, pt had acute visual disturbacnes and became agitated. She then proceeded to have 2 GTC Sz. – All studies were WNL incl CBC, Uric acid levels, plts, electrolytes as well as CT head, MRI, and LP. Eclampsia- with Sz on PPD 22? • Akerman and Hall site a couple influencial articles that prehaps challenge the way we traditionally think of eclampsia of occurring btwn 20 wks and 48hr postpartum. One larger retrospective study (from the US, Lubarsky, Barton, Freidman, Nasreddine, Ramadan, Sibia reported in 1994; 83, 502-505, in the green journal) reported that up to 15% of eclamptic pts developed sz btwn days 3 and 22 postpartum. Cont. • In another review (by Douglas and Redman of the UK in the BMJ 1994; 309;1395-1400) found that in over 300 cases of eclampsia, 12% occurred more than 48hr and 2% more than 7 days postpartum. – Among these cases reported, up to 90% suffered from HA and visual disturbances prior to sz and no classical preeclamptic signs were present in over 50% of the cases. The Big Picture… • The main objective of this article was to raise one major point…that far too often it is assumed by our colleagues that most postnatal HA’s are caused by our epidurals. It is this unwillingness to consider all possible causes for postnatal HA’s (other than PDPH) which may lead to a delay in the exclusion or diagnosis of more serious causes of HA’s – Including, but not limited to, cortical vein thrombosis, SAH or meningoencephalitis. How clearly can you see through mud? • And, as is many aspests of medicine, it is never a clear-cut matter. Thus, it is imperative to collaborate with colleagues (neurologists, OB, ID, radiologists) and make a conjoined effort to properly diagnose and treat each case appropriately. Aseptic meningitis secondary to chemical irritation. • “chemical meningitis” is an aseptic meningitis with • it’s acute clinical course and standard laboratory findings mimicking bacterial meningitis. Most commonly it’s a rare complication of giving dyes for myelography – There has been case reports of chemical meningitis following intrathecal or epidural corticosteroid thaerapies – Also following PO TMP/SMX, ibuprofen, celecoxib and rofecoxib More cases of chemical meningitis: • In Neurosurgery 55(5): 1222, November 2004, Meyers et • al. reported two cases of chemical meningitis secondary to the placement of second-generation aneurysm coils for treatment of large cerebral aneurysms. In Neurosurgery 25 (2): 264-270, August 1989, Lunardi et al reported a case of chemical meningitis resulting from spillage of the contents of a cystic cranial tumor. He also reviewed some 35 different cases in which cranial and spinal tumors were associated with a chemical meningitis. How do we avoid the sequelea that can originate from a dural puncture? • Should we always use LOR technique with NS and • • not air? Should we consider threading intrathecal catheters in cases where we accidentally puncture the dura while attempting placement of an epidural catheter? Should we prophylactically place EBP in parturients after inadvertent dural puncture? - Let’s investigate… LOR techniques…does it matter? • One larger study involving over 3700 pts investigated • upon the role of intrathecal air. In the journal Anesthesiolgy, 1998; 88; 76-81, Aida et al from Japan formed two investigative groups– 1,918 in the saline LOR group and 1,812 in the air LOR group – Epidurals were performed for chronic and acute pain purposes – Incidence, onset time, and duration of PDPH were examined and compared in each of the two groups. Cont. • Results: incidence of PDPH in the air group (32 cases) was • • significantly higher than that in the saline group (5 cases) ((more than 6 times more likely)), although the occurrences of meningeal perforation btwn the two groups did not differ- 48 cases of unintentional dural puncture in the air group vs 51 cases in the saline group. Also, PDPH’s were significantly more rapid in onset and shorter in duration in the air group vs the NS. Lastly, of the 32 cases of PDPH in the air group, 30 of them had intrathecal air bubbles (highly contributing to HA) detected on brain CT, whereas no intrathecal air bubbles were seen in the NS group Conclusion: • That perhaps the use of air for loss-ofresistance techniques used when performing epidural blocks, may be associated with a higher incidence of PDPH as this article suggests. Thread ‘em Subarachnoid? • Placement of a subarachnoid catheter after • unintentional dural puncture may reduce the incidence of PDPH. This is a contraversial issue, but shown to be the case in the following study. From Regional Anesthesia and Pain Management, Vol 28, No 6 (Nov-Dec) 2003, 512-515, Ayad et al. from CCF over a five year period, retrospectively investigated 115 pts who had unintentional dural punctures whom were placed randomly into 3 groups. Methods Cont. • Group A- had an epidural catheter placed at another interspace • Group B- had a subarachnoid catheter placed for labor analgesia that was removed immediately after delivery • Group C- had a subarachnoid catheter placed for labor analgesia that was removed 24 hrs after delivery Results: • The incidence of PDPH in the various groups were as follows… – Group A- 81% – Group B- 31% – Group C- 3% Whereas the overall incidence of PDPH after inadvertent dural puncture was 46.9% overall. This risk of developing a PDPH is reported to be as high as 75% in OB pts after unintentional dural puncture with a 16-18 G needle. Note: the type of epidural needle was not specified The avoidance technique… • In the journal of Anesthesiology, 2004; 101: 1422-1427 Scavone et al performed a randomized double blind study to access the effect of prophylactic epidural blood patches on the incidence of PDPH. Methods: • 64 parturients who incurred an accidental dural puncture were randomized into two groups – 1 group: would randomly receive a prophylactic epidural blood patch with 20 cc autologous blood – 2nd group: was the sham group Pt’s were followed daily watching for the development of a PDPH for a min of 5 days. Results: • 18 of 32 pts in each group (56%) developed a PDPH. Therapeutic blood patches were recommended in subjects with moderate HA (in those who reported trouble with caring for their children) and in those who reported severe HA. – The groups showed no significant difference in time of onset of PDPH, median peak pain scores, and numbers of days spent unable to care for their children Results Cont. • The conclusion of the study showed that in this group of pts, prophylactic EBP did not decrease the incidence of PDPH or that there was a statistical significance in outcome btwn the two groups. Therefore, the need to provide prophylactic EBP was not supported. In Conclusion… • I have reviewed a case report here today with the • • intent of addressing other potential causes of peripartum HA and seizures that one must consider when working up a pt with these findings. Differential diagnosis considerations… Lastly, we must always be constantly self evaluating, searching for new data which may support or negate ways that we practice so that we can best serve our pts.