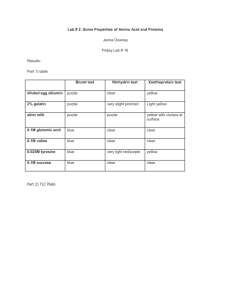
File
advertisement
COURSE TITLE: MEDICAL BIOCHEMISTRY TOPIC: PROTEIN METABOLISM LECTURER: MR. BELLO T. O. Among the classifications in amino acids, there is a form of classification that is based on their metabolism where there are glucogenic and ketogenic amino acids Glucogenic amino acids yield the precursors (keto acids) for the synthesis of carbohydrates i.e. arginine, phenylalanine e.t.c Ketogenic amino acids yield the precursors (ketone compounds like acetone,acetoacetate & β-hydroxyl butyric acid )for the synthesis of biomolecules like lipids. i.e leucine OVERVIEW OF AMINO ACIDS METABOLISM Metabolism has two parts; catabolism and anabolism Catabolism of amino acids Degradation of amino acids with some of the rxns. (oxidative deamination, transamination, decarboxylation) to give Keto acids (carbon skeleton) and ammonia Fates of carbon skeleton - gluconeogenesis, non essensial amino acids Fates of ammonia - Urea cycle for its excretion, assimilation into keto acids for non essential amino acids synthesis. Why is ammonia toxic? Metabolism of some amino acids, their specialized products and their metabolic disorder. Glycine 1. Synthesis of glycine: Synthesized from serine in the presence of hydroxymethyl transferase which is tetrahydrofolate dependent. Can be obtained from threonine, catalysed by threonine aldolase Glycine can be synthesized from one carbon compound (N5N10-methylene THF),CO2 and NH3 in the precence of glycine synthase. 2. Its catabolic product can actively be involved in the synthesis of many specialized product like Heme, purines, creatine e. t. c. SYNTHESIS OF SPECIALIZED PRODUCTS FROM GLYCINE Formation of purine ring: utilized in the formation of position 4 and 5 of carbon and position 7 nitrogen in the purine ring. Synthesis of heme (porphyrine ): glycine condenses with succinyl CoA to form δ-amino levulinate which is the precursor for Heme synthesis. Succinyl CoA +glycine -δ-amino levulinate (ALA) Biosynthesis of creatine:glycine, methionine and arginine are required for the synthesis of creatinine Transfer of guanidino group of arginine to glycine in the presence of arginine transamidase to produce guanidoacetate (glycocyamine). S-Adenosylmethionine (active methionine) transfers methyl group to glycocyamine to produce creatine which occurs in the liver. Creatine is phosphorylated to phosphocreatine (creatine phosphate) in the presence of creatine kinase and stored in the muscles as a high energy compound. Metabolic disorders of glycine metabolism: Glycinuria: This is a defect in which excretion of glycine is above 0.5 – 1 g/day due to defective renal reabsorption Primary hyperoxaluria: This defect is due to glycine transaminase impairment in glyoxalate oxidation to formate. It is charactcetrized by increased urinary oxalate leading to oxalate stones (oxalosis) Deposition of oxalate is observed in various tissues. PHENYLALANINE AND TYROSINE : Metabolism of phenylalanine occurs through tyrosine Tyrosine can be incorporated into various proteins like epinephrine, norepinephrine, dopamine (catecholamine), thyroid hormones and pigments like melanin. Conversion of phenylalanine to tyrosine: Degradation of phenyalanine occurs through tyrosine. Phenylalanine is hydroxylated by phenylalanine hydroxylase (present in the liver) to produce tyrosine. This is irreversible reaction and requires biopterine as the coenzyme. (tetrahydrobiopterine) which is oxidised to dihydrobiopterine, then regenerated by NADPH dependent dihydrobiopterine reductase. The failure or blockage of phenyalanine hydroxylase may result in phenylketonuria. Tyrosine undergoes transamination to give phydroxyphenylpyruvate in the presence of tyrosine transaminase (PLP dependent). p-hydroxyphenylpyruvate undergoes oxidative decarboxylation in presence of p-hydroxyphenylpyruvate oxidase or dioxygenase (copper containing enzyme) to produce homogentisate Homogentisate is converted to 4-maleylacetoacetate a reaction catalysed by homogentisate oxygenase which requires molecular oxygen to break the aromatic ring. Isomerization of 4-maleylacetoacetate to 4 fumarylacetoacetate reaction catalysed by maleylacetoacetate isomerase. 4 fumarylacetoacetate undergoes hydrolysis to yield fumarate and acetoacetate which are the precursor in lipid synthesis, TCA cycle and glucose synthesis. SYNTHESIS OF SPECIALIZED PRODUCTS FROM TYROSINE 1. MELANIN: Synthesis of melanin occurs in the melanosomes present in the melanocytes. Tyrosine is the precusor for melanin and tyrosinase (copper containing) is the primary enzyme that is involved in the synthesis of the pigment. Tyrosine gets converted to 3,4 – dihydroxyphenylalanine (DOPA) in the reaction catalysed by tyrosinase . DOPA gets converted to dopaquinone and in subsequent reactions it forms leucodopachrome, followed by 5,6 dihydroxyindole. Oxidation process takes place, Tyrosinase is involved in the sequential enzymatic conversion of 5,6 dihydroxyindole to indole quinone. Melanochrome is synthesized from indole quinone which on polymerization produces melanin. Alternatively, dopaquinone is condensed with cysteine and red melanin is generated. 2. BIOSYNTHESIS OF THYROID HORMONES: Thyroid hormones are synthesized from tyrosine. Tetraiodothyronine (thyroxine) and triiodothyronine hormones. Iodinization of tyrosine ring occurs to produce mono and diiodotyrosine from which triiodothyronine (T3) and thyroxine (T4) are synthesized Protein thyroglobulin undergoes proteolytic breakdown to release the free T3and T4 hormones. Disorders of tyrosine and phenylalanine metabolism: 1. PHENYLKETONURIA: Due to defective enzyme phenylalanine hydroxylase, this leads to accumulation of phenylalanine, which by transamination is converted to phenylpyruvate in tissues and blood. Elevated amount of phenylpyruvate and other keto acids are then found in urine. Biochemical manifestation: (i) Effect on CNS Mental retardation, failure to walk, failure to grow, tremor and failure to transport other aromatic amino acid like tryptophan and tyrosine and this leads to defect in myelin formation. (ii) Effect on pigmentationInhibition of tyrosinase leads to albinism Treatment: Dietary intake of phenylalanine should be measured in plasma levels and adjusted. Diagnosis: Done by Guthrie test and ferric chloride test and green colour is obtained. Normal values in phenylalanine in plasma PKU 20-65mg/dl 2. TYROSINEMIA TYPE II: (RICHNER-HANART SYNDROME) Due to blockage of tyrosine transaminase hence accumulation and excretion of tyrosine and its metabolites Characterised in skin (dermatitis) and eye lession ALKAPTONURIA: Defect on the enzyme homogentisate oxidase, hence accumulation of homogentisate in blood and tissues. Alkapton is the pigment produced, in case of accumulation occurs in the connective tissues, bones and various organs resulting in ochronosis Diagnosis: Benedict’s test,carry out ferric chloride and silver nitrate test in urine Treatment: Consumption of proteins with low phenylalanine contents TYOSINOSIS OR TYROSINEMIA TYPE I: Due to deficiency of Fumarylacetoacetate hydroxylase and maleylacetoacetate isomerase which may lead to liver failure, rickets, renal tubular dysfunction and polyneuropathy Treatment: Recommended diets with low tyrosine, phenylalanine and methionine ALBINISM: Causes: Deficiency or lack of enzyme tyrosinase Decrease in melanosomes of melanocytes Impairment in melanin polymerization Lack of protein matrix in melanosomes Limitation of substrate (tyrosine) availability Presence of inhibitors of tyrosinase The common cause of albinism is defect on tyrosinase, enzyme responsible for the synthesis of melanin. Clinical manifestation: Melanin protect the body from sun rays hence albinos are sensitive to sunlight and susceptible to skin cancer (carcinoma). Photophobia is associated with lack of the pigment in the eyes. UREA CYCLE: Site of metabolism is liver and urea is the end product of protein metabolism as elucidated by Hans kreb and Kurt Henseleit in 1932 Urea has two amino groups, one from ammonia and other from aspartate and carbon is supplied from carbon dioxide. Enzymes involved: Two enzymes are found in mitochondria while others are cytosolic. Steps in urea cycle: Synthesis of carbamoyl phosphate: Synthesized by condensation of carbon dioxide (CO2) with ammonium ions (NH4+) to form carbamyl phosphate in the presence of carbamoyl phosphate synthetase, a rate limiting enzyme requiring N-Acetylglutamate. Formation of citrulline: Citrulline is synthesized when ornithine is condensed with carbamoyl phosphate in the presence of ornithine transcarbamoylase. The citrulline synthesized at this point is transported into cytosol. Ornithine and citrulline are basic amino acids. Synthesis of arginosuccinate: Citrulline is condensed with aspartate in the presence of arginosuccinate synthetase to yield arginosuccinate. This is a step for second amino group incorporation and ATP is required and is cleaved to yield AMP and Ppi Cleavage of arginosuccinate: Arginosuccinate is cleaved by arginosuccinase to give fumarate and arginine. Arginine is immediate precursor for urea synthesis. Fumarate enters into TCA cycle, gluconeogenesis. Formation of urea: Arginase cleaves arginine to yield urea and ornithine. Ornithine enters into mitochondria for re-use. Arginase is activated by Mn2+ UREA CYCLE CLINICAL SIGNIFICANCE OF UREA A moderately active man consuming about 300 gm carbohydrates, 100 gm of fats and 100 gm of proteins daily must excrete about 16.5 gm of N daily. 95 per cent is eliminated by the kidneys and the remaining 5 percent, for the most part as N, in the faeces. 1. Normal Level The concentration of urea in normal blood plasma from a healthy fasting adult ranges from 20 to 40mg%. 2. Increase of Levels Increases in blood urea may occur in a number of diseases in addition to those in which the kidneys are primarily involved. The causes can be classified as: Prerenal Most important are conditions in which plasma vol/ body-fluids are reduced: Salt and water depletion, Severe and protracted vomiting as in pyloric and intestinal obstruction, Severe and prolonged diarrhoea, Pyloric stenosis with severe vomiting, Haematemesis, Haemorrhage and shock; shock due to severe burns, Ulcerative colitis with severe chloride loss, In crisis of Addison’s disease (hypoadrenalism). Renal The blood urea can be increased in all forms of kidney diseases: In acute glomerulonephritis. In early stages of type II nephritis (nephrosis) the blood urea may not be increased, but in later stages with renal failure, blood urea rises. Other conditions are malignant nephrosclerosis, chronic pyelonephritis and mercurial poisoning. In diseases such as hydronephrosis, renal tuberculosis; small increases are seen but depends on extent of kidney damage. Postrenal Diseases These lead to increase in blood urea, when there is obstruction to urine flow. This causes retention of urine and so reduces the effective filtration pressure at the glomeruli; when prolonged, produces irreversible kidney damage. Causes: Enlargement of prostate, Stones in urinary tract, Stricture of the urethra, Tumours of the bladder affecting urinary flow. 3. Decreased levels: Decreases in blood urea levels are rare. It may be seen: In some cases of severe liver damage, Physiological condition: Blood urea has been seen to be lower in pregnancy than in normal nonpregnant women. REGULATION OF UREA CYCLE: The rate limiting enzyme is carbamoyl phosphate synthetase I. Requires NAG (N-acetyl glutamate) synthesized from acetyl CoA and Glutamate. Increase of NAG increases synthesis of urea in the liver. Carbamoylphosphate synthetase I and Glutamate dehydrogenase located in the mitochondria coordinate the synthesis of NH3 for the synthesis of carbamoyl phosphate. The remaining four enzymes particitipate in the synthesis of urea depend on the concentration of the substrates. INTEGRATION OF UREA CYCLE WITH TCA CYCLE: The formation of fumarate in urea cycle is the integrating point to TCA cycle. Oxaloacetate undergoes transamination to produce aspartate which enters urea cycle as the second source of amino group to synthesis urea. ATP (12) generated in the TCA cycle while 4 ATP are utilized in urea cycle. CO2 and H2O are the end product that are formed on complete oxidation of various metabolites whereby CO2 generated is utilized in the urea synthesis. Disorder Enzyme involved Hyperammonemia type 1 Carbamoyl phosphate synthetase I Hyperammonemia type II Ornithine transcarbamoylase Citrullinemia Arginosuccinate synthase Arginosuccinemia Arginosuccinase Hyperargininemia Arginase Blood urea clinical significance: Pre-renal: is associated with increased protein breakdown hence negative nitrogen balance observed during diabetic coma, thyroxicosis, leukemia bleeding disorders Renal-increased: in cases of patients suffering from acute glomerulonephritis, chronic nephritis, nephrosclerosis,polycystic kidney. Post renal-elevated: in cases of urinary tract obstruction i.e. tumor, stones THE END