Treatment of Epilepsy
advertisement

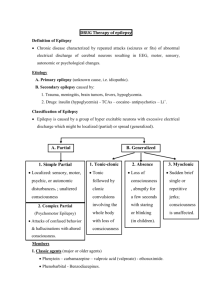
Academic Half-Day Treatment of Epilepsy Ruba Benini & Abdullah Tawakul July 25th , 2012 Preamble Epilepsy is the second most common neurological condition after headache Worldwide prevalence of 1% with a cumulative incidence of 2-4%. The incidence of epilepsy is highest in the very young and the very old. Hauser et al., 1996 Preamble Epilepsy is not a single disease entity but rather an umbrella term used to denote a variety of disorders with different etiologies but with seizures as a common denominator Port Wine Stain (Sturge-Weber) Mesial Temporal lobe sclerosis Prosencephaly Preamble Treatment of epilepsy can be broadly divided into: Medical treatment (anticonvulsants) Surgical treatment (Focal resections; Hemispherectomy; Callosotomy) Special diets (Ketogenic diet, Atkinson diet) Other (Vagal Nerve stimulation, Deep brain stimulation, Transcranial Magnetic Stimulation) OUTLINE Approach to a first unprovoked seizure – to treat or not to treat Adult versus Child Medical Treatment What anticonvulsants are available to you Mechanisms of action Some important pharmacokinetic properties to keep in mind Some dos and don’ts Surgical Treatment Brief overview Others A few words OUTLINE Approach to a first unprovoked seizure – to treat or not to treat Adult versus Child Medical Treatment What anticonvulsants are available to you Mechanisms of action Some important pharmacokinetic properties to keep in mind Some dos and don’ts Surgical Treatment Brief overview Others A few words Scenario 25 year old male, presents to the ER for an episode this morning where he was found on the bathroom floor by his girlfriend after she heard a big bang. Consult says: r/o seizure. What is a seizure? What do you want to know? How do you take a seizure history? Scenario 25 year old male, presents to the ER for an episode this morning where he was found on the bathroom floor by his girlfriend after she heard a big bang. Consult says: r/o seizure. What is a seizure? What do you want to know? How do you take a seizure history? Definitions What is a Seizure: •Clinical event characterized by transient neurological signs and/or symptoms (motor, sensory, level of consciousness) •That arise due to abnormal and excessive discharges from hyperexcitable, synchronized neuronal networks Scenario 25 year old male, presents to the ER for an episode this morning where he was found on the bathroom floor by his girlfriend after she heard a big bang. Consult says: r/o seizure. What is a seizure? What do you want to know? How do you take a seizure history? Approach to a first Seizure • Is this really an epileptic seizure HISTORY! or a seizure mimic? HISTORY! • What type of seizure was it? (Seizure Semiology) HISTORY! • Can you identify a particular epilepsy syndrome? • What is the etiology of the seizure? Approach to a first Seizure • Is this really an epileptic seizure or a seizure mimic? Suggested Reading: Crompton and Berkovic (2009) The borderland of epilepsy: clinical and molecular features of phenomena that mimic epileptic seizures. Lancet Neurology Approach to a first Seizure • What type of seizure was it? Focal (Partial) Seizures Generalized seizures •Simple partial •Complex partial •Complex partial with secondary generalization •Tonic-clonic (Grand mal) •Absence (Petit mal) •Myoclonic •Tonic •Clonic •Atonic Simple partial seizures No loss of consciousness May manifest as motor signs, autonomic symptoms, somatosensory, special sensory symptoms or psychic symptoms Complex partial seizures Impairment of consciousness Usually originate in frontal or temporal lobe Maybe preceded by auras May involve automatisms Approach to a first Seizure • What type of seizure was it? Suggested Reading: Berg et al. (2010) Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005– 2009. Epilepsia. Approach to a first Seizure • Can you identify a particular epilepsy syndrome? Suggested Reading: Berg et al. (2010) Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005– 2009. Epilepsia. Definitions What is an Epilepsy Syndrome: Clinical entity with relatively consistent clinical features that is defined by seizure semiology, etiology, EEG signature, neurologic status, prognosis and in some cases response to specific anticonvulsants Approach to a first Seizure • Can you identify a particular epilepsy syndrome? Suggested Reading: Berg et al. (2010) Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005– 2009. Epilepsia. Approach to a first Seizure • What is the etiology? Back to Scenario 25 year old male, presents to the ER for an episode this morning where he was found on the bathroom floor by his girlfriend after she heard a big bang. Consult says: r/o seizure. First unprovoked Seizure Generalized seizure Patient asks if he has epilepsy? Do you treat? Definitions What is Epilepsy: Chronic condition characterized by recurrent, usually spontaneous, epileptic seizures Two or more unprovoked seizures Back to Scenario 25 year old male, presents to the ER for an episode this morning where he was found on the bathroom floor by his girlfriend after she heard a big bang. Consult says: r/o seizure. First unprovoked Seizure Generalized seizure Patient asks if he has epilepsy? Do you treat? Approach to first unprovoked seizure •Risk of recurrence after first seizure: 30 to 55% over 2 to 5 years •Treatment of first seizure reduces risk of recurrence by 50% but does not alter the risk of developing epilepsy •There is no evidence that delaying treatment alters prognosis (chances for eventual seizure control are not reduced by delaying AED therapy) Approach to first unprovoked seizure First unprovoked epileptic seizure No treatment Exceptions: Early treatment is justifiable for patients in whom recurrence of seizure would have significant consequences related to driving, working and general safety Summary of Guideline 1. Treatment with AED is not indicated for the prevention of the development of epilepsy (Level B). 2. Treatment with AED may be considered in circumstances where the benefits of reducing the risk of a second seizure outweigh the risks of pharmacologic and psychosocial side effects (Level B). Hirtz et al., 2003 OUTLINE Approach to a first unprovoked seizure – to treat or not to treat Adult versus Child Medical Treatment What anticonvulsants are available to you Mechanisms of action Some important pharmacokinetic properties to keep in mind Some dos and don’ts Surgical Treatment Brief overview Others A few words Treatment of Epilepsy (Anticonvulsants) In epilepsy, there is a pathologic imbalance between inhibitory and excitatory processes Excitation Inhibition Anticonvulsants control seizures either by increasing inhibition or decreasing excitation •Voltage-gated Na channels •Voltage-gated Ca channels •GABAergic transmission •Glutamatergic excitation Excitation Inhibition Treatment of Epilepsy (Anticonvulsants) Treatment of Epilepsy (Anticonvulsants) Mechanism of action Important side-effects Pharmacokinetics How do you choose the first drug Special considerations (pregnancy, etc) Match the following anticonvulsants to their mechanism(s) of action Anticonvulsants (Mechanism of Action) Blocks voltage-gated Na channels Blocks presynaptic release of neurotransmitter by blocking SV 2A Blocks presynaptic release of neurotransmitter by N-type Ca channels Blocks GAT-1 and prevents uptake of GABA from synapse Phenytoin (Dilantin) GABA(A) receptor agonist Increases intracellular GABA levels Blocks voltage-gated Ca channels Blocks T-type Calcium channels Stabilizes slowly-inactivated voltage-gated Na channels Blocks NMDA receptors Blocks AMPA receptors Blocks metabolism of GABA by inhibiting GABA-T Anticonvulsants (Mechanism of Action) Blocks voltage-gated Na channels Blocks presynaptic release of neurotransmitter by blocking SV 2A Blocks presynaptic release of neurotransmitter by N-type Ca channels Blocks GAT-1 and prevents uptake of GABA from synapse Phenobarbital GABA(A) receptor agonist Increases intracellular GABA levels Blocks voltage-gated Ca channels Blocks T-type Calcium channels Stabilizes slowly-inactivated voltage-gated Na channels Blocks NMDA receptors Blocks AMPA receptors Blocks metabolism of GABA by inhibiting GABA-T Anticonvulsants (Mechanism of Action) Blocks voltage-gated Na channels Blocks presynaptic release of neurotransmitter by blocking SV 2A Blocks presynaptic release of neurotransmitter by N-type Ca channels Blocks GAT-1 and prevents uptake of GABA from synapse Carbamazepine (Tegretol) GABA(A) receptor agonist Increases intracellular GABA levels OxCarbamazepine (Trileptal) Blocks voltage-gated Ca channels Blocks T-type Calcium channels Stabilizes slowly-inactivated voltage-gated Na channels Blocks NMDA receptors Blocks AMPA receptors Blocks metabolism of GABA by inhibiting GABA-T Anticonvulsants (Mechanism of Action) Blocks voltage-gated Na channels Blocks presynaptic release of neurotransmitter by blocking SV 2A Blocks presynaptic release of neurotransmitter by N-type Ca channels Blocks GAT-1 and prevents uptake of GABA from synapse Clobazam (Frisium) GABA(A) receptor agonist Increases intracellular GABA levels Diazepam Lorazepam Midazolam Clonazepam Blocks voltage-gated Ca channels Blocks T-type Calcium channels Stabilizes slowly-inactivated voltage-gated Na channels Blocks NMDA receptors Blocks AMPA receptors Blocks metabolism of GABA by inhibiting GABA-T Anticonvulsants (Mechanism of Action) Blocks voltage-gated Na channels Blocks presynaptic release of neurotransmitter by blocking SV 2A Blocks presynaptic release of neurotransmitter by N-type Ca channels Blocks GAT-1 and prevents uptake of GABA from synapse Lamotrigine (Lamictal) GABA(A) receptor agonist Increases intracellular GABA levels Blocks voltage-gated Ca channels Blocks T-type Calcium channels Stabilizes slowly-inactivated voltage-gated Na channels Blocks NMDA receptors Blocks AMPA receptors Blocks metabolism of GABA by inhibiting GABA-T Anticonvulsants (Mechanism of Action) Blocks voltage-gated Na channels Blocks presynaptic release of neurotransmitter by blocking SV 2A Blocks presynaptic release of neurotransmitter by N-type Ca channels Blocks GAT-1 and prevents uptake of GABA from synapse Levetiracetam (Keppra) GABA(A) receptor agonist Increases intracellular GABA levels Blocks voltage-gated Ca channels Blocks T-type Calcium channels Stabilizes slowly-inactivated voltage-gated Na channels Blocks NMDA receptors Blocks AMPA receptors Blocks metabolism of GABA by inhibiting GABA-T Anticonvulsants (Mechanism of Action) Blocks voltage-gated Na channels Blocks presynaptic release of neurotransmitter by blocking SV 2A Blocks presynaptic release of neurotransmitter by N-type Ca channels Blocks GAT-1 and prevents uptake of GABA from synapse Lacosamide (Vimpat) GABA(A) receptor agonist Increases intracellular GABA levels Blocks voltage-gated Ca channels Blocks T-type Calcium channels Stabilizes slowly-inactivated voltage-gated Na channels Blocks NMDA receptors Blocks AMPA receptors Binds to CRMP-2 Anticonvulsants (Mechanism of Action) Blocks voltage-gated Na channels Blocks presynaptic release of neurotransmitter by blocking SV 2A Blocks presynaptic release of neurotransmitter by N-type Ca channels Blocks GAT-1 and prevents uptake of GABA from synapse Valproic Acid (Epival, Depakene) GABA(A) receptor agonist Increases intracellular GABA levels Blocks voltage-gated Ca channels Blocks T-type Calcium channels Stabilizes slowly-inactivated voltage-gated Na channels Blocks NMDA receptors Blocks AMPA receptors Blocks metabolism of GABA by inhibiting GABA-T Anticonvulsants (Mechanism of Action) Blocks voltage-gated Na channels Blocks presynaptic release of neurotransmitter by blocking SV 2A Blocks presynaptic release of neurotransmitter by N-type Ca channels Blocks GAT-1 and prevents uptake of GABA from synapse Valproic Acid (Epival, Depakene) GABA(A) receptor agonist Increases intracellular GABA levels Blocks voltage-gated Ca channels Blocks T-type Calcium channels Stabilizes slowly-inactivated voltage-gated Na channels Blocks NMDA receptors Blocks AMPA receptors Blocks metabolism of GABA by inhibiting GABA-T Anticonvulsants (Mechanism of Action) Blocks voltage-gated Na channels Blocks presynaptic release of neurotransmitter by blocking SV 2A Blocks presynaptic release of neurotransmitter by N-type Ca channels Blocks GAT-1 and prevents uptake of GABA from synapse Ethosuximide (Zarontin) GABA(A) receptor agonist Increases intracellular GABA levels Blocks voltage-gated Ca channels Blocks T-type Calcium channels Stabilizes slowly-inactivated voltage-gated Na channels Blocks NMDA receptors Blocks AMPA receptors Blocks metabolism of GABA by inhibiting GABA-T Anticonvulsants (Mechanism of Action) Blocks voltage-gated Na channels Blocks presynaptic release of neurotransmitter by blocking SV 2A Blocks presynaptic release of neurotransmitter by N-type Ca channels Blocks GAT-1 and prevents uptake of GABA from synapse Topiramate (Topamax) GABA(A) receptor agonist Increases intracellular GABA levels Blocks voltage-gated Ca channels Blocks T-type Calcium channels Stabilizes slowly-inactivated voltage-gated Na channels Blocks NMDA receptors Blocks AMPA (non-NMDA) receptors Blocks metabolism of GABA by inhibiting GABA-T Anticonvulsants (Mechanism of Action) Blocks voltage-gated Na channels Blocks presynaptic release of neurotransmitter by blocking SV 2A Blocks presynaptic release of neurotransmitter by N-type Ca channels Blocks GAT-1 and prevents uptake of GABA from synapse Vigabatrin (Sabril) GABA(A) receptor agonist Increases intracellular GABA levels Blocks voltage-gated Ca channels Blocks T-type Calcium channels Stabilizes slowly-inactivated voltage-gated Na channels Blocks NMDA receptors Blocks AMPA receptors Blocks metabolism of GABA by inhibiting GABA-T Anticonvulsants (Mechanism of Action) Blocks voltage-gated Na channels Blocks presynaptic release of neurotransmitter by blocking SV 2A Blocks presynaptic release of neurotransmitter by N-type Ca channels Blocks GAT-1 and prevents uptake of GABA from synapse Tiagabine GABA(A) receptor agonist Increases intracellular GABA levels Blocks voltage-gated Ca channels Blocks T-type Calcium channels Stabilizes slowly-inactivated voltage-gated Na channels Blocks NMDA receptors Blocks AMPA receptors Blocks metabolism of GABA by inhibiting GABA-T Anticonvulsants (Mechanism of Action) Blocks voltage-gated Na channels Blocks presynaptic release of neurotransmitter by blocking SV 2A Blocks presynaptic release of neurotransmitter by N-type Ca channels Blocks GAT-1 and prevents uptake of GABA from synapse Gabapentin (Neurontin) GABA(A) receptor agonist Increases intracellular GABA levels Pregabalin (Lyrica) Blocks voltage-gated Ca channels Blocks T-type Calcium channels Stabilizes slowly-inactivated voltage-gated Na channels Blocks NMDA receptors Blocks AMPA receptors Blocks metabolism of GABA by inhibiting GABA-T Anticonvulsants (Mechanism of Action) Blocks voltage-gated Na channels Blocks presynaptic release of neurotransmitter by blocking SV 2A Blocks presynaptic release of neurotransmitter by N-type Ca channels Blocks GAT-1 and prevents uptake of GABA from synapse Felbamate GABA(A) receptor agonist Increases intracellular GABA levels Blocks voltage-gated Ca channels Blocks T-type Calcium channels Stabilizes slowly-inactivated voltage-gated Na channels Blocks NMDA receptors Blocks AMPA receptors Blocks metabolism of GABA by inhibiting GABA-T Anticonvulsants (Mechanism of Action) Blocks voltage-gated Na channels Blocks presynaptic release of neurotransmitter by blocking SV 2A Blocks presynaptic release of neurotransmitter by N-type Ca channels Blocks GAT-1 and prevents uptake of GABA from synapse Rufinimide GABA(A) receptor agonist Increases intracellular GABA levels Blocks voltage-gated Ca channels Blocks T-type Calcium channels Stabilizes slowly-inactivated voltage-gated Na channels Blocks NMDA receptors Blocks AMPA receptors Blocks metabolism of GABA by inhibiting GABA-T Summary Panayiotopoulos (2010) Anticonvulsants: Summary Drug Mechanism of Action Phenobarbital Agonist of GABA (A) receptors Antagonist of N- and L-type voltage-gated Ca channels Phenytoin Stabilizes inactive state of voltage-gated Na Channels Inhibit presynaptic release of NT via L-type Ca channels Carbamazepine Oxcarbazepine Stabilizes inactive state of voltage-gated Na Channels Inhibit presynaptic release of NT via L-type Ca channels Valproate Stabilizes inactive state of voltage-gated Na Channels Increases GABA levels Blocks NMDA glutamate receptors Blocks T-type voltage gated Ca channels Ethosuximide Antagonist of T-type voltage-gated Calcium channels Benzodiazepines (clobazam) Agonist of GABA (A) receptors Anticonvulsants: Summary Drug Mechanism of Action Lamotrigine Stabilizes inactive state of voltage-gated Na Channels Increases intracellular GABA levels May act at N, P/Q type voltage-gated Calcium channels Vigabatrin Blocks metabolism of GABA through GABA-T Gabapentin Pregabalin Blocks presynaptic release of neurotransmitters via N-type Calcium channels Increases intracellular GABA levels Tiagabine Blocks GAT-1 and prevents uptake of GABA from synapse Felbamate Blocks NMDA glutamate receptors Enhances GABA(A) receptor transmission Unclear effect on voltage-gated Na channels Levetiracetam Blocks presynaptic vesicle recycling through SV 2A Anticonvulsants: Summary Drug Mechanism of Action Lacosamide Stabilizes slowly-inactivated Na channels Binds to CRMP-2 Topiramate Blocks AMPA/Kainate glutamate receptors Blocks L-type voltage gated Ca channels Unclear effect on voltage-gated Na channels May enhance GABA(A) receptor transmission Weak inhibitor of carbonic anhydrase Anticonvulsants and side-effects PART I: What makes nerve cells excitable? Anticonvulsants: Side-effects Which of the following AED can cause somnolence? Phenobarbital Valproic acid Topiramate Vigabatrin Phenytoin Lamictal Primidone Clobazam PART I: What makes nerve cells excitable? Anticonvulsants: Side-effects Which of the following AED can cause somnolence? Phenobarbital Valproic acid Topiramate Vigabatrin Phenytoin Lamictal Primidone Clobazam PART I: What makes nerve cells excitable? Anticonvulsants: Side-effects Which of the following are side-effects of Topiramate? 1. Blurry vision 2. Metabolic acidosis 3. Paresthesias 4. Ataxia 5. Renal stones 6. Mental slowing with speech and memory disturbance 7. Psychosis 8. Alopecia 9. Weight gain 10. Glaucoma PART I: What makes nerve cells excitable? Anticonvulsants: Side-effects Which of the following are side-effects of Topiramate? 1. Blurry vision 2. Metabolic acidosis 3. Paresthesias 4. Ataxia 5. Renal stones 6. Mental slowing with speech and memory disturbance 7. Psychosis 8. Alopecia 9. Weight gain 10. Glaucoma PART I: What makes nerve cells excitable? Anticonvulsants: Side-effects Which of the following are side-effects of Phenytoin? 1. Blurry vision 2. Metabolic acidosis 3. Paresthesias 4. Ataxia 5. Renal stones 6. Hirsutism 7. Psychosis 8. Osteoporosis 9. Alopecia 10. Weight gain 11. Gum hyperplasia 12. Stevens-Johnson syndrome 13. Blood dyscrasias PART I: What makes nerve cells excitable? Anticonvulsants: Side-effects Which of the following are side-effects of Phenytoin? 1. Blurry vision 2. Metabolic acidosis 3. Paresthesias 4. Ataxia 5. Renal stones 6. Hirsutism 7. Psychosis 8. Osteoporosis 9. Alopecia 10. Weight gain 11. Gum hyperplasia 12. Stevens-Johnson syndrome 13. Blood dyscrasias PART I: What makes nerve cells excitable? Anticonvulsants: Side-effects Which of the following are side-effects of Valproic acid? 1. Blurry vision 2. Dysfunctional platelets 3. Paresthesias 4. Ataxia 5. Birth defects 6. Hirsutism 7. Psychosis 8. Abdominal pain and other GI symptoms 9. Alopecia 10. Weight gain 11. Liver failure 12. Stevens-Johnson syndrome 13. Blood dyscrasias PART I: What makes nerve cells excitable? Anticonvulsants: Side-effects Which of the following are side-effects of Valproic acid? 1. Blurry vision 2. Dysfunctional platelets 3. Paresthesias 4. Ataxia 5. Birth defects 6. Hirsutism 7. Psychosis 8. Abdominal pain and other GI symptoms 9. Alopecia 10. Weight gain 11. Liver failure 12. Stevens-Johnson syndrome 13. Hyperammonemia PART I: What makes nerve cells excitable? Anticonvulsants: Side-effects Which of the following are side-effects of Carbamazepine? 1. 2. 3. 4. 5. 6. 7. 8. Ataxia SJS Visual field loss SIADH Diplopia Hepatotoxicity Aplastic anemia Paresthesias PART I: What makes nerve cells excitable? Anticonvulsants: Side-effects Which of the following are side-effects of Carbamazepine? 1. 2. 3. 4. 5. 6. 7. 8. Ataxia SJS Visual field loss SIADH Diplopia Hepatotoxicity Aplastic anemia Paresthesias PART I: What makes nerve cells excitable? Anticonvulsants: Side-effects Which of the following anticonvulsants cause peripheral visual field defects? 1. 2. 3. 4. 5. 6. Tegretol Phenobarbital Vigabatrin Clobazam Valproic acid Dilantin PART I: What makes nerve cells excitable? Anticonvulsants: Side-effects Which of the following anticonvulsants cause peripheral visual field defects? 1. 2. 3. 4. 5. 6. Tegretol Phenobarbital Vigabatrin Clobazam Valproic acid Dilantin Bonus Point: What type of seizures is Vigabatrin used for? PART I: What makes nerve cells excitable? Anticonvulsants: Side-effects Which of the following anticonvulsant(s) can be used in patients with concomitant psychiatric disorders for mood stabilization? 1. 2. 3. 4. 5. 6. 7. 8. Levetiracetam Phenobarbital Oxcarbazepine Vigabatrin Clobazam Valproic acid Dilantin Lamotrigine PART I: What makes nerve cells excitable? Anticonvulsants: Side-effects Which of the following anticonvulsant(s) can be used in patients with concomitant psychiatric disorders for mood stabilization? 1. 2. 3. 4. 5. 6. 7. 8. Levetiracetam Phenobarbital Oxcarbazepine Vigabatrin Clobazam Valproic acid Dilantin Lamotrigine PART I: What makes nerve cells excitable? Anticonvulsants: Side-effects Which of the following anticonvulsant(s) can cause SJS 1. Levetiracetam 2. Phenobarbital 3. Oxcarbazepine 4. Vigabatrin 5. Clobazam 6. Carbamazepine 7. Phenytoin 8. Ethosuximide 9. Valproic acid 10. Felbamate 11. Lamotrigine 12. Lacosamide PART I: What makes nerve cells excitable? Anticonvulsants: Side-effects Which of the following anticonvulsant(s) can cause SJS 1. Levetiracetam 2. Phenobarbital 3. Oxcarbazepine 4. Vigabatrin 5. Clobazam 6. Carbamazepine 7. Phenytoin 8. Ethosuximide 9. Valproic acid 10. Felbamate 11. Lamotrigine 12. Lacosamide Anticonvulsants: Side-effects Continuum (2010) Which of the following anticonvulsants need to be monitored and why? 1. Phenytoin 2. Phenobarbital 3. Clobazam 4. Topamax 5. Carbamazepine 6. Oxcarbamazepine 7. Levetiracetam 8. Valproic acid 9. Gabapentin 10. Pregabalin 11. Ethosuximide 12. Lacosamide 13. Vigabatrin Anticonvulsants and Pharmacokinetics PART I: What makes nerve cells excitable? Anticonvulsants: Pharmacokinetics All of the following anticonvulsants are hepatically excreted except: 1. 2. 3. 4. 5. 6. 7. 8. Phenobarbital Oxcarbazepine Carbamazepine Phenytoin Levetiracetam Valproic acid Lamotrigine Lacosamide PART I: What makes nerve cells excitable? Anticonvulsants: Pharmacokinetics All of the following anticonvulsants are hepatically excreted except: 1. 2. 3. 4. 5. 6. 7. 8. Phenobarbital Oxcarbazepine Carbamazepine Phenytoin Levetiracetam Valproic acid Lamotrigine Lacosamide Anticonvulsants: Pharmacokinetics Panayiotopoulos (2010) PART I: What makes nerve cells excitable? Anticonvulsants: Pharmacokinetics Which of the following antoconvulsants decrease efficacy of OCP? 1. 2. 3. 4. 5. 6. 7. 8. Carbamazepine/Oxcarbazepine Phenobarbital Valproic acid Topiramate Vigabatrin Phenytoin Lamictal Primidone PART I: What makes nerve cells excitable? Anticonvulsants: Pharmacokinetics Which of the following antoconvulsants decrease efficacy of OCP? 1. 2. 3. 4. 5. 6. 7. 8. Carbamazepine/Oxcarbazepine Phenobarbital Valproic acid Topiramate Vigabatrin Phenytoin Lamictal (level decreases with OCP use) Primidone http://basic-clinical-pharmacology.net/chapter%2024_%20antiseizure%20drugs.htm PART I: What makes nerve cells excitable? Anticonvulsants: Pharmacokinetics Enzyme-Inducers: •Increase rate of metabolism of drugs metabolized by CYP enzymes •Results in changes in sex hormone levels and increases clearance of estrogen and progesterone in OCP •Increase metabolism of Vit D (which is metabolized by liver) → rickets and hypocalcemia in children Panayiotopoulos (2010) PART I: What makes nerve cells excitable? Anticonvulsants: Pharmacokinetics Which of the following anticonvulsants will be increased with the concomitant use of erythromycin or clarithromycin? 1. 2. 3. 4. 5. 6. 7. 8. Carbamazepine Phenobarbital Valproic acid Topiramate Vigabatrin Phenytoin Lamictal Primidone PART I: What makes nerve cells excitable? Anticonvulsants: Pharmacokinetics Which of the following anticonvulsants will be increased with the concomitant use of erythromycin or clarithromycin? 1. 2. 3. 4. 5. 6. 7. 8. Carbamazepine Phenobarbital Valproic acid Topiramate Vigabatrin Phenytoin Lamictal Primidone PART I: What makes nerve cells excitable? Anticonvulsants: Pharmacokinetics A patient who drinks lots of grapefruit juice presents with toxic levels of which of the following anticonvulsants: 1. 2. 3. 4. 5. 6. 7. 8. Carbamazepine Phenobarbital Valproic acid Topiramate Vigabatrin Phenytoin Lamictal Primidone PART I: What makes nerve cells excitable? Anticonvulsants: Pharmacokinetics A patient who drinks lots of grapefruit juice presents with toxic levels of which of the following anticonvulsants: 1. 2. 3. 4. 5. 6. 7. 8. Carbamazepine (grapefruit inhibits CYP3A4) Phenobarbital Valproic acid Topiramate Vigabatrin Phenytoin Lamictal Primidone PART I: What makes nerve cells excitable? Anticonvulsants: Pharmacokinetics Anticonvulsants (phenytoin, phenobarbital) can generally have the following effect on warfarin: 1. Increase warfarin level 2. Decrease warfarin level PART I: What makes nerve cells excitable? Anticonvulsants: Pharmacokinetics Anticonvulsants (phenytoin, phenobarbital) can generally have the following effect on warfarin: 1. Increase warfarin level 2. Decrease warfarin level PART I: What makes nerve cells excitable? Anticonvulsants: Pharmacokinetics The pharmacokinetics of phenytoin can be described as: 1. 2. 3. 4. Non-linear Linear First-order Zero-order PART I: What makes nerve cells excitable? Anticonvulsants: Pharmacokinetics The pharmacokinetics of phenytoin can be described as: 1. 2. 3. 4. Non-linear Linear First-order Zero-order PART I: What makes nerve cells excitable? Anticonvulsants: Pharmacokinetics An ICU patient with multiple medical problems is on IV dilantin for the treatment of status epilepticus. What lab variable do you need to order to ascertain the correct dilantin level? 1. 2. 3. 4. 5. Liver enzymes Albumin CBC Urea Ammonia PART I: What makes nerve cells excitable? Anticonvulsants: Pharmacokinetics An ICU patient with multiple medical problems is on IV dilantin for the treatment of status epilepticus. What lab variable do you need to order to ascertain the correct dilantin level? 1. 2. 3. 4. 5. Liver enzymes Albumin CBC Urea Ammonia Special considerations PART I: What makes nerve cells excitable? Anticonvulsants: Special Considerations You just diagnosed a 16 year old girl with JME. Which of the following drugs would you not prescibe? 1. Lamictal 2. Levetiracetam 3. Valproic acid PART I: What makes nerve cells excitable? Anticonvulsants: Special Considerations You just diagnosed a 16 year old girl with JME. Which of the following drugs would you not prescibe? 1. Lamictal 2. Levetiracetam 3. Valproic acid PART I: What makes nerve cells excitable? Anticonvulsants: Special Considerations Choosing among anticonvulsants Anticonvulsants: Choosing AEDs Which of the following are important to consider when choosing an anticonvulsant 1. 2. 3. 4. 5. 6. Age Type of seizure (partial versus generalized) Patient characteristics Co-morbid conditions Cost Side-effect profile Anticonvulsants: Choosing AEDs Which of the following are important to consider when choosing an anticonvulsant 1. 2. 3. 4. 5. 6. Age Type of seizure (partial versus generalized) Patient characteristics Co-morbid conditions Cost Side-effect profile Anticonvulsants: Choosing AEDs High-level evidence for the efficacy for initial monotherapy of partial seizures exists for which of the following drugs: 1. 2. 3. 4. 5. 6. 7. Phenytoin Lamotrigine Phenobarbital Carbamazepine Oxcarbazepine Topiramate Valproic acid Anticonvulsants: Choosing AEDs High-level evidence for the efficacy for initial monotherapy of partial seizures exists for which of the following drugs: 1. 2. 3. 4. 5. 6. 7. Phenytoin Lamotrigine Phenobarbital Carbamazepine Oxcarbazepine Topiramate Valproic acid Anticonvulsants: Choosing AEDs High-level evidence for the efficacy for initial monotherapy of generalized seizures exists for which of the following drugs: 1. 2. 3. 4. 5. 6. 7. Phenytoin Lamotrigine Phenobarbital Carbamazepine Oxcarbazepine Topiramate Valproic acid Anticonvulsants: Choosing AEDs High-level evidence for the efficacy for initial monotherapy of generalized seizures exists for which of the following drugs: 1. 2. 3. 4. 5. 6. 7. Phenytoin Lamotrigine Phenobarbital Carbamazepine Oxcarbazepine Topiramate Valproic acid Anticonvulsants: Choosing AEDs 8 year old girl with childhood absence epilepsy, your choice(s) include: 1. 2. 3. 4. 5. 6. 7. Phenytoin Ethosuximide Phenobarbital Lamictal Oxcarbazepine Topiramate Valproic acid Anticonvulsants: Choosing AEDs 8 year old girl with childhood absence epilepsy, your choice(s) include: 1. 2. 3. 4. 5. 6. 7. Phenytoin Ethosuximide Phenobarbital Lamictal Oxcarbazepine Topiramate Valproic acid Anticonvulsants: Choosing AEDs 16 year old boy with JME, your choice(s) include all of the following except: 1. 2. 3. 4. Lamictal Carbamazepine Valproic acid Levetiracetam Anticonvulsants: Choosing AEDs 16 year old boy with JME, your choice(s) include all of the following except: 1. 2. 3. 4. Lamictal Carbamazepine Valproic acid Levetiracetam PART I: What makes nerve cells excitable? Anticonvulsants: Summary Panayiotopoulos (2010) OUTLINE Approach to a first unprovoked seizure – to treat or not to treat Adult versus Child Medical Treatment What anticonvulsants are available to you Mechanisms of action Some important pharmacokinetic properties to keep in mind Some dos and don’ts Surgical Treatment Brief overview Others A few words Introduction Approximately 20% to 30% of all patients with epilepsy will have physically, socially, and medically refractory seizure disorders. Patients with intractable epilepsy are at increased risk for serious morbidity and mortality. Introduction The goals of therapy in patients with medically refractory seizures include: significantly reducing seizure tendency. avoiding adverse effects. permitting the individual to become a participating and productive member of society. WHEN TO DECIDE DRUG THERAPY HAS FAILED DRE is now defined as ‘failure of adequate trials of two tolerated, appropriately chosen and used AED schedules (whether as monotherapy or in combination) to achieve sustained seizure freedom. less than 5% to 10%, who have not responded to monotherapy with two appropriate antiepileptic drugs (AEDs) or a combination of two drugs will respond to a third drug. after treatment with multiple AEDS, 11% and 16% became seizure free. It is interesting that 52% of patients treated surgically in one of these studies became seizure free. Who should be referred for surgery Surgery outcome Pre-surgical evaluation Conclusion Epilepsy surgery is highly effective and has durable benefits, and improves quality of life. Despite class I evidence and Clinical Practice Guidelines, epilepsy surgery remains underutilized. The spectrum of patients who may benefit from epilepsy surgery has expanded considerably including younger and older patients and those without apparent MRI lesions. Electronic Devices VNS. Direct Cortical Stimulation. Responsive Neurostimulation System. Stimulation of the Anterior Nucleus of the Thalamus for Epilepsy Trial. VNS Vagus nerve stimulation(VNS) is an approved treatment for intractable partial epilepsy. A VNS is a palliative procedure that may reduce seizure tendency. In clinical trials, seizure frequency was reduced by 25% to 30%, an outcome similar to new AED trials. Day surgery procedure. The left vagus nerve is stimulated rather than the right because the right plays a role in cardiac function, and stimulating it could have negative cardiac effects. Mechanism : Unknown , affect blood flow to different parts of the brain and to affect neurotransmitters, including serotonin and norepinephrine, which are implicated in depression. common side effects include hoarseness, throat pain, cough, dyspnea and paresthesia. THE KETOGENIC DIET The ketogenic diet was developed before all of the anticonvulsants in current use except phenobarbital. It fell out of favor with the introduction of phenytoin. Since 1990 ,the ketogenic diet has resurfaced as it is often very effective in patients who have failed numerous drug trials. patients on the diet often require lower doses of anticonvulsants and become more alert and less dizzy. Description and Mechanism The classic diet consists of 4 grams of fat for each gram of protein and carbohydrate consumed. Mechanism : Unknown ,overall changes in brain protein phosphorylation state and particular examples of altered gene expression have been documented. Early proposals suggesting that cerebral acidosis or changes in electrolyte concentrations are responsible for the diet’s anti seizure effects Efficacy The ketogenic diet is a first-line therapy for patients with seizures associated with certain metabolic disorders. A number of studies have shown the ketogenic diet to be an effective treatment for medically intractable epilepsy in children. One of the prospective studies included 51 children from 1 to 8 years of age. At the 12-month follow-up, 10% of the children were seizure free, 22% had a greater than 90% reduction in seizure frequency, and 40% had a greater than 50% reduction. With adolescents , 45 patients aged 12 to 19 years, 20 patients remained on the diet at 1 year. Seven had a 50% to 90% reduction in seizure frequency, while six had a greater than 90% reduction. One recent adult study. This study included 11 adults between age of 32 -45. At 8 months follow-up, three patients had a 90% decrease in seizure frequency, three patients had a 50% to 89% decrease ,and one patient had a less than 50%decrease. 6 patients discontinued the diet. 2 had no change in their seizure frequency . Side Effects and Precaution Common adverse effects in adolescent and adult trials Included constipation, hypercholesterolemia, Menstrual irregularities, and weight loss. Kidney stones occur in 6% to7% of children on the diet. Because valproate is an inhibitor of fatty acid oxidation and decreases hepatic ketogenesis, Valproate is not recommended. PART I: What makes nerve cells excitable? References: Deckers et al. Conference Report. Current limitations of antiepileptic drug therapy:a conference review. Epilepsy Research 53 (2003) 1–17. Joana Guimara˜es, and Jose´ Augusto Mendes Ribeiro. Pharmacology of Antiepileptic Drugs in Clinical Practice. The Neurologist 2010;16:353–357. Johannessen SI, Landmark CJ. Antiepileptic drug interactions - principles and clinical implications. Curr Neuropharmacol. 2010 Sep;8(3):254-67. Panayiotopoulos CP. A Clinical Guide to Epileptic Syndromes and Their treatment. Second Edition. 2010. Continuum. Epilepsy. 2010. http://basic-clinical-pharmacology.net/chapter%2024_%20antiseizure%20drugs.htm PART I: What makes nerve cells excitable? Questions?? Figure 1. Focal seizures result from a limited group of neurons that fire abnormally because of intrinsic or extrinsic factors. (a) In this simplified diagram, II and III represent epileptic neurons. Because of extensive cell-to-cell connections, termed 'recurrent collaterals', aberrant activity in cells II and III can fire synchronously, resulting in a prolonged depolarization of the neurons. (b) This intense depolarization of epileptic neurons is termed the paroxysmal depolarization shift. The prolonged depolarization results in action potentials and propagation of electrical discharges to other cells. The paroxysmal depolarization shift is largely dependent Anticonvulsants: Voltage-gated Na channels •Blockade/modulation of Voltage-gated Na channels is the most common mechanism of action of most of the AEDs •Bind and stabilize inactive forms of channel → prevent repetitive neuronal firing CBZ PHT VPA Oxcarbazepine ? Eslicarbazepine Felbamate LTG Topiramate Zonisamide Lacosamide Rufinamide Approach to Epilepsy (When to stop treatment) •Seizure control is possible for most patients with epilepsy •In a recent study by Brodie et al. (2012, Neurology) •69% seizure-freedom over 2 years •61% seizure-freedom over 5 years •52% seizure-freedom over 10 years •After many years of seizure-freedom, patient begin to question whether therapy is still necessary •Desire to discontinue medications: •Side-effects •Cost •Inconvenience •Fear of long-term side-effects •Risk of recurrence after anticonvulsant discontinuation: 12% to 63% over 2 to 5 years follow-up (majority: 41% or less relapse rate) Approach to Epilepsy (When to stop treatment) Approach to Epilepsy (When to stop treatment)