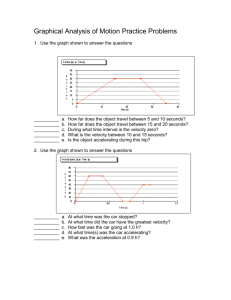
Stoker Boiler Model
advertisement
Compressible Flow Introduction Objectives: 1. Indicate when compressibility effects are important. 2. Classify flows with Mach Number. 3. Introduce equations for adiabatic, isentropic flows. Larry Baxter Ch En 374 Flow Classifications V Ma a Flow Regime Ma 0.3 Density Gradient Incompressible Negligible Shock Waves None 0.3 Ma 0.8 Subsonic Small None 0.8 Ma 1.2 Transonic Significant First appear 1.2 Ma 3.0 Supersonic Significant Significant Dominant Dominant 3.0 Ma Hypersonic Property Changes dp dh dp Tds dh ds T T 2 2 2 dh dp 1 ds 1 T R 1 p 2 s 1 c p dT T p2 cv dT 2 R ln R ln p1 1 T 1 2 For isentropic (Δs=0), constant-heat-capacity conditions p2 T2 p1 T1 k /( k 1) k cp 2 ; k cv 1 Speed of Sound C p1, 1,T1,V1 0 p2, 2,T2,V2 0 p1, 1,T1,V1 C C p2, 2,T2,V2 C V pressure wave Δx=nλ 1AV1 2 AV2 V C 1 Fright m V2 V1 1AC V p1A p2 A p 2 1 p 1CV 1C C 1 1 2 Speed of Sound in Materials p p lim C a 0 2 1/ 2 kp a 1/ 2 p a kRT 1/ 2 1/ 2 s Most (perfect) gas conditions High frequency waves (isothermal rather than isentropic expansion) RT 1/ 2 1/ 2 K p K a s Solids and liquids (actually gases as well), where K is bulk modulus Bulk modulus, not heat capacity ratio Typical Sound Speeds (STP) Gas Air Ar C3H6 1117 1038 1009 mi/hr 762 708 688 C3H8 810 552 247 CH4 1447 987 441 CO CO2 1136 869 775 593 346 265 H2 4236 2888 1291 H2O 1381 941 421 He N2 3280 1136 2236 775 1000 346 O2 1061 723 323 238 299 204 91 UF6 ft/s Liquid Benzene Carbon Tetrachloride Ethanol Glycerin Kerosene Machine Oil Mercury Water, fresh Water, salt ft/s 4340 3080 3810 6102 4390 4240 4757 4888 4990 m/s 341 316 307 mi/hr 2959 2100 2598 4161 2993 2891 3244 3333 3402 m/s 1323 939 1161 1860 1338 1292 1450 1490 1521 Solid Aluminum Beryllium Brass Brick Concrete Copper Cork Glass Gold Iron Hickory Ice Lead Platinum Rubber Steel Wood ft/s 16896 42290 11401 13701 10600 12799 1312 12999 10630 19521 13189 10499 3799 10696 328 19554 12999 mi/hr 11520 28834 7773 9341 7228 8726 895 8863 7248 13310 8992 7158 2590 7292 224 13332 8863 m/s 5150 12890 3475 4176 3231 3901 400 3962 3240 5950 4020 3200 1158 3260 100 5960 3962 Generally, sound travels faster in solids than liquids and faster in liquids than gases. Sound Speed vs. Molecular Speed Molecular theory of gases indicates that the average molecular speed is 1/ 2 3p 1/ 2 2 2 2 2 3RT c c x c y c z c Therefore, the average velocity of a molecule (speed in any specified direction) is 1 2 c c RT c x RT 3 In the case of a sound wave, molecules don’t have time to adjust their temperatures to the rapid change in pressure, so their temperature changes slightly inside the wave. If this change is completely adiabatic – generally a good assumption – the specific heat ratio accounts for the temperature change. Thus, the speed of sound is identically equal to the speed at which molecules travel in any one direction under conditions of a propagating wave. 2 x Sound Travels in Longitudinal Waves Light, cello strings, and surfing waves are transverse waves. Sound travels in a longitudinal or compression wave. Ideal and Perfect Gases Ideal Gas p RT c p cv R Good approximation for most conditions far from critical points and at atmospheric pressure or lower. Perfect Gas c p c p (T ) Reasonable approximation for many gases. Generally also assume that the gas is noncp k k (T ) dissociating. cv Gas Flows V12 V22 h1 gz1 h2 gz2 q w v 2 2 V12 V22 h1 h2 const h0 c pT0 2 2 1/ 2 Perfect Gas Vmax 2c pT0 T0 V2 1 2c pT0 T 2 k a c pT R T k 1 k 1 V 2 k 1 T0 1 2 2a T Mach-Number Relations T0 k 1 2 1 Ma T 2 1/ 2 a0 T0 a T p0 T0 p T k 1 2 1 Ma 2 k /( k 1) 1/( k 1) 0 T0 T 1/ 2 k 1 2 1 Ma 2 k 1 2 1 Ma 2 k /( k 1) Isentropic Expansion 1 /( k 1) Isentropic Expansion Graphical Representation stagnation/static property 20 T0/T p0/2000T rho0/100T a0/a 15 10 5 0 0 2 6 4 Mach Number 8 10 Critical Properties T* 2 T0 k 1 a* 2 a0 k 1 0.8333 for k =1.4 (air) 1/ 2 p* 2 p0 k 1 0.9129 for k =1.4 (air) k /( k 1) * 2 0 k 1 0.5283 for k =1.4 (air) 1/( k 1) 0.6339 for k =1.4 (air) Blunt Body Flows Ma = 2.2 Sonic Flows Ma = 1.7 Ma = 3.0 Compressible Flow Essentials • Know what a Mach number is and the regimes of flow as indicated by the Mach number. (Mach number is ratio of velocity to the speed of sound at the same conditions. Mach numbers of 0.3, 0.8, 1.2, and 3 separate incompressible, subsonic, transonic, supersonic, and hypersonic regimes, respectively). • Know how pressure, temperature, density, and velocity change across a normal shock wave. (First three all increase in direction of decreasing velocity, with pressure increasing the most. Velocity decreases from supersonic to subsonic value, with post-shock velocity decreasing as pre-shock velocity increases). Supersonic vs. Subsonic Flows ( x )V ( x )A( x ) const d dV dA 0 V A dp VdV 0 dp a d 2 dV dA 1 dp 2 V A 1 Ma V 2 Area Changes Differ with Ma Critical Area ( x )V ( x )A( x ) const A V* A* * V k 1 2 1 Ma A 1 2 A * Ma k 1 2 k 1 2( k 1) Mass Flow Relationships Choked flow 1/( k 1) 1/ 2 2 m *max * A * V * 0 k 1 2k A* RT0 k 1 0.6847 p0 A * 1/ 2 m *max (k 1.4) 0.6847 A * 0 RT0 1/ 2 RT0 All flows 1/ 2 2k p m RT0 A p0 k 1 p0 2/k p 1 p0 ( k 1) / k 1/ 2 Normal Shock Wave Shock Waves p2 1 2kMa12 (k 1) p1 k 1 Ma22 k 1Ma12 2 2kMa12 (k 1) 2 (k 1)Ma12 V1 2 1 (k 1)Ma1 2 V2 T2 2kMa12 (k 1) 2 (k 1)Ma1 2 T1 k 12 Ma12 T0,2 1 T0,1 0,2 p0,2 (k 1)Ma 0,1 p0,1 (k 1)Ma12 2 2 1 k /( k 1) k 1 2 2 kMa ( k 1 ) 1 1/( k 1) Normal Shock Wave Nozzle Performance Compressible Flow Essentials • Be able to explain on a molecular level the origin of the changes in pressure, temperature and density. (Molecules collide into one another or a surface, exchanging kinetic energy for pressure or temperature. Ideal gas law still applies to give relationship between density, pressure, and temperature). • Know how streamlining designs differ for compressible flows compared to incompressible flows. (Leading edges are relatively sharp edges rather than rounded corners and heat dissipation is a major issue). Three Classes of CFD • Finite Difference • Original and still widely used formulation for CFD describes flow fields as values of velocity vectors at discrete points. • Finite Volume • Close cousin to finite difference, but discrete points represent average values of velocities in a volume rather than at a point. • Finite Element • Most commonly used for heat transfer and stress calculations in solid bodies rather than fluid mechanics (because of stability issues). • Much easier to describe general/complex geometries than FD/FV techniques. • Solves for dependent variable (velocity, temperature, stress) with variations across element by minimizing an objective function First Derivative FD Formulas ui 1 ui 1 ui 1 ui 1 x i 1 x i 1 2x central O(Δx2) ui ui 1 ui ui 1 x i x i 1 x backward O(Δx) ui 1 ui ui 1 ui x i 1 x i x forward O(Δx) 3ui 4ui 1 u j 2 2x 3ui 4ui 1 u j 2 2x backward O(Δx2) forward O(Δx2) First Derivative FV Formulas ui 1/ 2 ui 1/ 2 x General Formula u i 1/ 2 u i u i 1 / 2 central O(Δx2) u i 1/ 2 u i , u i 1/ 2 u i 1 backward O(Δx) u i 1/ 2 u i 1, u i 1/ 2 u i forward O(Δx) u i 1/ 2 u i 1/ 2 u i 1/ 2 u i 1/ 2 3u i u i 1 , 2 3u i 1 u i 2 2 3u i 1 u i 2 , 2 3u i u i 1 2 backward O(Δx2) forward O(Δx2) Second Derivative FD Formulas ui 1 2ui ui 1 2 x ui 2ui 1 ui 2 2 x ui 2ui 1 ui 2 x 2 central O(Δx2) backward O(Δx) forward O(Δx) First Derivative FV Formulas ui 1/ 2 ui 1/ 2 x General Formula ui 1/ 2 ui 1 ui / x, ui 1/ 2 ui ui 1 / x central O(Δx2) ui 1/ 2 ui ui 1 / x, backward O(Δx) ui 1/ 2 ui 1 ui 2 / x ui 1/ 2 ui 2 ui 1 / x, ui 1/ 2 ui 1 ui / x forward O(Δx) Navier-Stokes: Cartesian Coord. x component 2Vx 2Vx 2Vx Vx Vx Vx Vx p g x Vx Vy Vz 2 2 2 x y z x y z t x y component 2Vy 2Vy 2Vy Vy Vy Vy Vy p Vx Vy Vz 2 2 2 x y z y x y z t g y z component 2Vz 2Vz 2Vz Vz Vz Vz Vz p g z Vx Vy Vz 2 2 2 x y z z y z t x Outline of CFD model y Econo. Boiler Secondary air ~8 kg/s, 175 ºC Secondary air ~8 kg/s, 175 ºC x z Super heater #1 Super heater #2: 194 m2 / 2090 ft2 Super heater #1: 364 m2 / 3920 ft2 Boiler Bank: 1181 m2 / 12700 ft2 Economizer: 330 m2 / 3550 ft2 Super heater #2 Stoker: Geometry and Surface Areas Spreader stokers ~9 kg fuel/s Grate air ~24 kg/s, 175 ºC Computational mesh Cloud (Particle) Trajectories Oxygen Mass Fraction Contours Velocity and Heat Release Vary Initial Deposition Rates Vary Temporal Deposition Variation Gas Temperature Field CFD Essentials • Know the distinguishing characteristics of finite difference, finite volume, and finite element approaches to numerical methods differ. • Know where to find (in these notes) common algebraic approximations for first and second derivatives for FD and FV approaches and the accuracy of the approximation. • Know (conceptually) how the algebraic approximations are substituted into the partial differential equations and how these are then solved. • Recognize that entire careers are dedicated to small fractions of CFD problem solving because of issues of convergence, stability, non-uniform grids, turbulence, etc.