Pre-chem ch. 4 - CESA 10 Moodle
advertisement
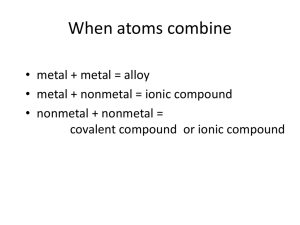
Nomenclature and formulas of compounds What a compound is and how structure affects properties of compounds Discuss differences and similarities between ionic, covalent, and metallic bonds. Name and write formulas for ionic and covalent compounds. Differentiate between alkane, alkene, and alcohol. Recognize polymers and relate them to your life. Mixture Compound Same chemical formula Chemical bond Chemical structure Bond length Bond angle Ball and stick Space filling Structural formula Ionic Metal and nonmetal Covalent nonmentals Metallic metals Gain and lose electrons Metal and nonmetal Conduct electricity when dissolved in water 2 nonmetals Sharing of electrons Polar Nonpolar Polyatomic ions Sea of electrons Name the cation first and the anion second. Monatomic cations use the element name. Monatomic anions take their name from the root of the element name plus the suffix –ide. If the compound contains a polyatomic in, simply name the ion. Li2O BeCl2 NaCl MgBr2 K2(SO4) (NH4)3PO4 Lithium oxide Beryllium chloride Magnesium bromide Potassium sulfate Ammonium phospate To figure out the charge of the transition metal Multiply the number of anions by the charge The cations overall charge will be equal to but opposite Divide the overall charge by the amount of cations This gives you the charge of each cation Put this number in roman numerals and in parenthesis TiN FeO Fe2O3 As2O5 SiI4 P4S3 PCl3 P4O10 Diarsenic pentoxide Silicion tetroxide Tetraphosphorus trisulfide Phosphorous trichloride Tetraphosphorous decoxide C based compounds Alkane End in –ane Single bonded carbons Methane Ethane Propane Alkene End in –ene Double bonded Ethene propene Alkyne Triple bonded C Propyne Butyne Alcohol End in –ol Have an –OH group on them Methanol Ethanol Propanol Polymers- large organic molecule made of many smaller bonded units Biochemical Compounds- organic compound that has an important role in living things carbohydrate proteins amino acid