JJ Thompson! - chem20-2011
advertisement

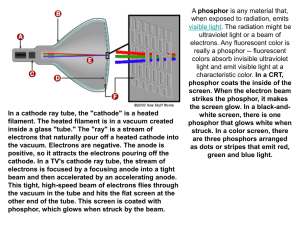
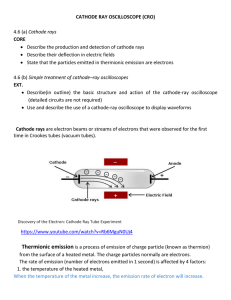
By: Elise, Erica and Camisha. Real name is Joseph John. Born in Cheetham Hill, Manchester. Born on December 18 1856. Died August 30 1940. His religion is Anglican He discovered the electron. He discovered the Electron in 1904. The chips are the electrons and the dough is a cloud of positive particles surrounding the chips(electrons). IDEAS OF THIS THEORY Atoms are a sphere with a positive charge distributed evenly through the atom containing negatively charged electrons separately embedded into the atom. Atoms as a whole are neutrally charged because the amount of positive charge in it cancels out the amount of negatively charged electrons. This means as a whole atoms are neutrally charged. Electrons can be moved around or taken out of the atom, the positive charge can not. Cathode Ray When investigating cathode rays using a highly evacuated discharge tube he was able to use the calculated velocity and deflection of the beam to calculate the ratio of electric charge to mass of the cathode ray. This was found to be constant regardless of the gas used in the tube and the metal of the cathode and was approximately 1000 times less than the value calculated for hydrogen ions in the electrolysis of liquids. J.J Thomson created this experiment in 1897. He did three different trials of this experiment. The first trial he determined how to build it with a metal cylinder at the end. The second trial he proved the rays carry a negative charge. The third trial he discovered that series of experiments can gradually uncover truths. Bibliography • http://www.nobelprize.org/nobel_prizes/physics/laureates/1906/t homson-bio.html • • http://www.aip.org/history/electron/jjhome.htm • • http://www.nndb.com/people/479/000099182/ • • http://en.wikipedia.org/wiki/J._J._Thomson • • http://www.experiment-resources.com/cathode-ray.html THE END!