Bioenergetics and Metabolism
advertisement

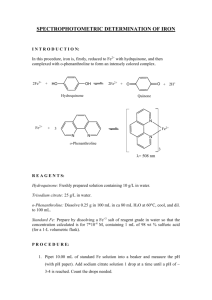
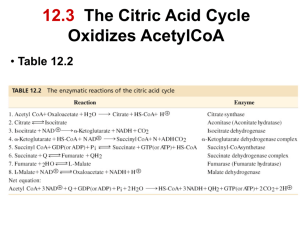
چرخه کربس مرکز سوخت و ساز سلولي مي شناسند ،چرا که افزون بر کوشندگي هاي سوخت و سوزي براي کربوهيدراتها ،فرآيند سوخت و ساز اسيدهاي چرب و اسيدهاي آمينه نيز وابسته به اين چرخه است The "currency exchange" for redox energy and ATP synthesis in the mitochondria electron transport chain is ~2.5 ATP/ NADH. Oxidation of 2 FADH2 molecules by the electron transport chain results in only ~3 molecules of ATP (~1.5 ATP/FADH2) because of differences in where these two coenzymes enter the electron transport chain. Based on this ATP currency exchange ratio, and the one substrate level phosphorylation reaction, each turn of the cycle produces ~10 ATP for every acetyl-CoA that is oxidized. Hans Krebs Elucidated the Citrate Cycle Hans Krebs, a biochemist who fled Nazi Germany for England in 1933, first described the citrate cycle in 1937. The citrate cycle is sometimes called the Krebs cycle, the citric acid cycle, or the tricarboxylic acid cycle, although we will refer to it as the citrate cycle because citrate is the first product of the pathway. The unprotonated form of citric acid is citrate which is the predominant species at physiological pH (the pKa values of the three carboxylate groups are 3.1, 4.7 and 6.4). Pathway Questions What does the citrate cycle accomplish for the cell? Transfers 8 electrons from acetyl-CoA to the coenzymes NAD+ and FAD to form 3 NADH and 1 FADH2 which are then reoxidized by the electron transport chain to produce ATP by the process of oxidative phosphorylation. Generates 2 CO2 as “waste products” and uses substrate level phosphorylation to generate 1 GTP which is converted to ATP by nucleoside diphosphate kinase. Supplies metabolic intermediates for amino acid and porphyrin biosynthesis. What is the overall net reaction of citrate cycle? Acetyl-CoA + 3 NAD+ + FAD + GDP + Pi + 2 H2O → CoA + 2 CO2 + 3 NADH + 2 H+ + FADH2 + GTP ΔGº’ = -57.3 kJ/mol What are the key regulated enzymes in citrate cycle? Pyruvate dehydrogenase – not a citrate cycle enzyme but it is critical to flux of acetyl-CoA through the cycle; this multisubunit enzyme complex requires five coenzymes, is activated by NAD+, CoA and Ca2+ (in muscle cells), and inhibited by acetyl-CoA, ATP and NADH. Citrate synthase – catalyzes the first reaction in the pathway and can be inhibited by citrate, succinyl-CoA, NADH and ATP; inhibition by ATP is reversed by ADP. What are the key regulated enzymes in citrate cycle? Isocitrate dehydrogenase - catalyzes the oxidative decarboxylation of isocitrate by transferring two electrons to NAD+ to form NADH, and in the process, releasing CO2, it is activated by ADP and Ca2+ and inhibited by NADH and ATP. α-ketoglutarate dehydrogenase - functionally similar to pyruvate dehydrogenase in that it is a multisubunit complex, requires the same five coenzymes and catalyzes an oxidative decarboxylation reaction that produces CO2, NADH and succinyl-CoA; it is activated by Ca2+ and AMP and it is inhibited by NADH, succinyl-CoA and ATP. Pathway Questions What are examples of citrate cycle in real life? Citrate is produced commercially by fermentation methods using the microorganism Aspergillus niger. Every year almost a half of million tonnes (5 x 108 kg) of citrate are produced worldwide by exploiting the citrate synthase reaction. Purified citrate is a food additive The complete oxidation of glucose to CO2 and H2O is summarized by the reaction: Glucose (C6H12O6) + 6O2 → 6CO2 + 6H2O ΔGº’ = -2,840 kJ/mol ΔG = -2,937 kJ/mol Four of the CO2 molecules are produced in the Citrate Cycle, but what reaction generates the other two CO2? Eight Reactions of the Citrate Cycle In the first half of the cycle, the two carbon acetate group of acetyl-CoA is linked to the four carbon oxaloacetate substrate to form a six carbon citrate molecule. Citrate is then converted to isocitrate to set up two decarboxylation reactions yielding two NADH and the high energy four carbon cycle intermediate succinylCoA. In the second half of the cycle, oxaloacetate is regenerated from succinyl-CoA by four successive reactions that lead to the formation of one GTP (ATP), one FADH2, and one NADH. Reaction 1: Condensation of oxaloacetate and acetyl-CoA by citrate synthase to form citrate This reaction commits the acetate unit of acetyl-CoA to oxidative decarboxylation Reaction follows an ordered mechanism: Oxaloacetate binds, inducing a conformational change in the enzyme that facilitates: - acetyl-CoA binding - formation of the transient intermediate, citryl-CoA - rapid hydrolysis that releases CoA-SH and citrate Reaction 2: Isomerization of citrate by aconitase to form isocitrate This is a reversible two step isomerization reaction. The intermediate, cis-aconitate, is formed by a dehydration reaction that requires the participation of an iron-sulfur cluster (4Fe-4S) in the enzyme active site. H2O is added back to convert the double bond in cis-aconitate, to a single bond with a hydroxyl group, on the terminal carbon. Reaction 3: Oxidative decarboxylation of isocitrate by isocitrate dehydrogenase to form α-ketoglutarate, CO2 and NADH First of two decarboxylation steps in the citrate cycle First reaction to generate NADH used for energy conversion reactions in the electron transport system Catalyzes an oxidation reaction that generates the transient intermediate oxalosuccinate In the presence of the divalent cations Mg2+ or Mn2+, oxalosuccinate is decarboxylated to form α-ketoglutarate Reaction 4: Oxidative decarboxylation of by α-ketoglutarate dehydrogenase to form succinyl-CoA, CO2 and NADH Second oxidative decarboxylation reaction and also produces NADH. α-Ketoglutarate dehydrogenase complex utilizes essentially the same catalytic mechanism we have already described for the pyruvate dehydrogenase reaction. Includes the binding of substrate to an E1 subunit (α-ketoglutarate dehydrogenase), followed by decarboxylation and formation of a TPP-linked intermediate. Reaction 5: Conversion of succinyl-CoA to succinate by succinyl-CoA synthetase in a substrate level phosphorylation reaction that generates GTP The available free energy in the thioester bond of succinyl-CoA (ΔGº' = -32.6 kJ/mol) is used in the succinyl-CoA synthetase reaction to carry out a phosphoryl transfer reaction (ΔGº' = +30.5 kJ/mol), in this case, a substrate level phosphorylation reaction, that produces GTP (or ATP). Nucleoside diphosphate kinase interconverts GTP and ATP by a readily reversible phosphoryl transfer reaction: GTP + ADP ↔ GDP + ATP (ΔGº' = 0 kJ/mol). Reaction 6: Oxidation of succinate by succinate dehydrogenase to form fumarate This coupled redox reaction directly links the citrate cycle to the electron transport system through the redox conjugate pair FAD/FADH2 which is covalently linked to the enzyme succinate dehydrogenase, an inner mitochondrial membrane protein. Oxidation of succinate results in the transfer of 2 e- to the FAD moiety, which in turn, passes the two electrons to the electron carrier coenzyme Q in complex II of the electron transport system. Reaction 6: Oxidation of succinate by succinate dehydrogenase to form fumarate Is FAD oxidized or reduced in this redox reaction? Is succinate the reductant or the oxidant in this reaction? Reaction 7: Hydration of fumarate by fumarase to form malate Fumarase the reversible hydration of the C=C double bond in fumarate to generate the L-isomer of malate. Fumarate and malate are citrate cycle intermediates that enter and exit the cycle from several different interconnected pathways. Reaction 8: Oxidation of malate by malate dehydrogenase to form oxaloacetate Oxidation of the hydroxyl group of malate to form oxaloacetate in a coupled redox reaction involving NAD+/NADH. The change in standard free energy for this reaction is unfavorable (ΔGº' = +29.7 kJ/mol), but the actual G for this reaction is favorable. In order for this unfavorable Gº’ to allow for a favorable G, the metabolite concentrations need to be far from equilibrium. Based on what you know about the citrate cycle, what do you think explains the favorable G in terms of [metabolite] in this case? Bioenergetics of the citrate cycle ?? Glycolysis + pyruvate dehydrogenase reaction + citrate cycle = net reaction: Glucose + 2 H2O + 10 NAD+ + 2 FAD + 4 ADP + 4 Pi → 6 CO2 + 10 NADH + 6 H+ + 2 FADH2 + 4 ATP