Chapter 2 - Colby College Wiki
advertisement
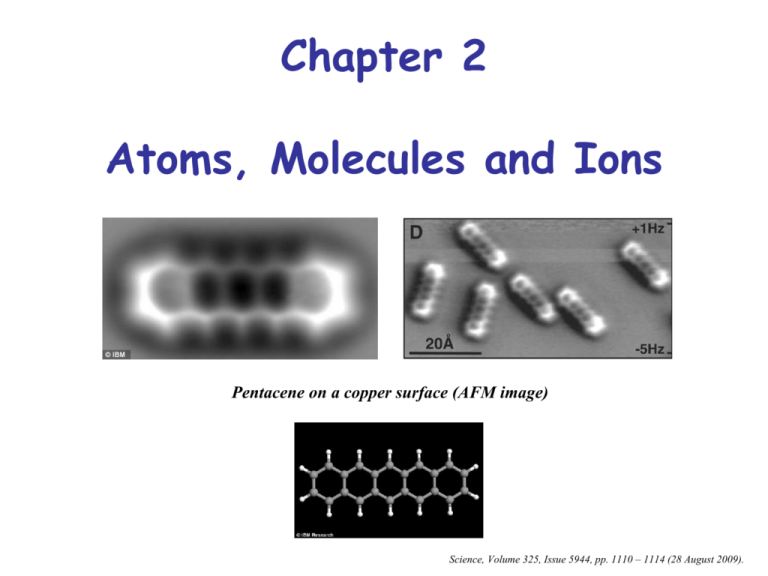
Chapter 2 Atoms, Molecules and Ions Pentacene on a copper surface (AFM image) Science, Volume 325, Issue 5944, pp. 1110 – 1114 (28 August 2009). (Dalton’s) Atomic Theory • Each element is made up of atoms. • Atoms of a given element are identical while atoms of different elements differ. • Chemical compounds are made up of specific whole number ratios of atoms. • Chemical Reactions involve the reorganization of atoms – atoms (and masses) do not change. John Dalton, 1766 -1844 Lithograph Properties of Charged Particles Sir Joseph John Thompson, 1856 - 1940 Thomson was awarded the 1906 Nobel Prize. An evacuated tube, containing a small amount of a gas was attached to a power supply: Electron mass/charge = -5.6857 x 10-9 g coulomb-1 • The same cathode rays (electrons) were seen no matter what gas the tube was filled with. • But the positively charged portion left over had a different mass/charge dependent on the type of gas used. Thompson’s Conclusion: Electrons are a fundamental unit of all materials, and atoms look like a “sea” of positive charge with imbedded electrons: Millikan’s Oil Drop Experiment Determined the charge on an electron = 1.592 X 10-19 coulombs Types of Radiation (Marie Curie/Rutherford) •-rays are high-energy light. • -particles are helium nuclei. • -particles are high energy electrons Marie Curie 1867-1934 •Met Pierre Curie, Physics Professor at the Sorbonne in 1894 •Succeeded her husband as Head of the Physics Laboratory at the Sorbonne •Following the death of Pierre Curie in 1906, she took his place as Professor of General Physics in the Faculty of Sciences, the first time a woman had held this position. •Received the Nobel Prize for Physics in 1903 •Received a second Nobel Prize in Chemistry in 1911 •Discovered two elements (Polonium and Radium) Marie Curie (Maria Sklodowska) 1867-1934 •Met Pierre Curie, Physics Professor at the Sorbonne in 1894 •Succeeded her husband as Head of the Physics Laboratory at the Sorbonne •Following the death of Pierre Curie in 1906, she took his place as Professor of General Physics in the Faculty of Sciences, the first time a woman had held this position. •Received the Nobel Prize for Physics in 1903 •Received a second Nobel Prize in Chemistry in 1911 •Discovered two elements (Polonium and Radium) Rutherford’s Gold Foil Experiment The Composition of the Atom Rutherford protons 1919 James Chadwick neutrons 1932 The nucleus is made up of protons and neutrons The “Modern” Atom The Fundamental Particles Particle Mass g Electron Proton Neutron 9.109 x 10-28 1.673 x 10-24 1.675 x 10-24 Charge amu (0) 1 1 Coulombs (e) –1.602 x 10-19 +1.602 x 10-19 0 –1 +1 0 Atoms are uncharged species – thus they contain the same number of protons and electrons (charged species are known as ions) The Concept of Atomic Number 12 6 C The Concept of Atomic Number 12 The atomic number (Z) = # of protons 6 C The Concept of Atomic Number The atomic mass (A) = protons + neutrons The atomic number (Z) = # of protons 12 6 C Atomic Mass Units The atomic mass (A) = protons + neutrons 12 6 -23 • Mass of carbon-12 = 1.9926 X 10 C g • Mass of carbon-12 = 12 amu (atomic mass units - amu or u) Isotopic Distributions Why does the periodic table say that the mass of carbon is 12.011 amu? Isotopic Distributions Why does the periodic table say that the mass of carbon is 12.011 amu? 98.9% of all carbon atoms have A = 12 (6 neutrons) 1.1% of all carbon atoms have A = 13 (7 neutrons) 12.011 is the average mass of a large sample of carbon atoms. When we measure “large” amounts of atoms, we can always use the average atomic mass. Mass Spectromeric Isotope Indentification Mass Spectrometric Isotope Identification 202Hg 200Hg 199Hg 201Hg 198Hg 204Hg 196Hg How many atoms are in 1 g of copper? How many atoms are in 1 g of copper? If Cu has an atomic mass of 63.546 amu, how does this easily convert to grams? Avogadro’s Number (the mole) relates atomic mass to sample mass: NA = 6.022 X 1023 = 1 mole 1 mole of atoms = mass in grams equal to the atomic mass How many atoms are in 1 g of copper? If Cu has an atomic mass of 63.546 amu, how does this easily convert to grams? Avogadro’s Number (the mole) relates atomic mass to sample mass: NA = 6.022 X 1023 = 1 mole 1 mole of atoms = mass in grams equal to the atomic mass The molar mass, M, is the mass of one mole of a substance. Ions and Ionic Compounds A charged species is called an ion. Metals tend to lose electrons to form positively charged ions – cations. Nonmetals tend to gain electrons to form negatively charged ions – anions. When forming compounds, the metal and nonmetal ions combine so that the net charge on a formula unit is zero. 23 11 Na + The atomic charge = protons – electrons Ions and Ionic Compounds Ionic compounds are held together by the electrostatic attraction of opposite charges - this is also known as coulombic attraction. This attraction is an ionic bond. Ionic compounds behave as an aggregate, not individual molecules Predicting Ionic Charges Main group elements tend to lose or gain electrons in order to equal the electron count of the nearest noble gas Predicting Ionic Charges Main group elements tend to lose or gain electrons in order to equal the electron count of the nearest noble gas Transition Metals Are Able To Form Multiple Cations: +1, +2, +3 and/or +4





