Struktur von SrFeO2.5 - Universität Paderborn
advertisement
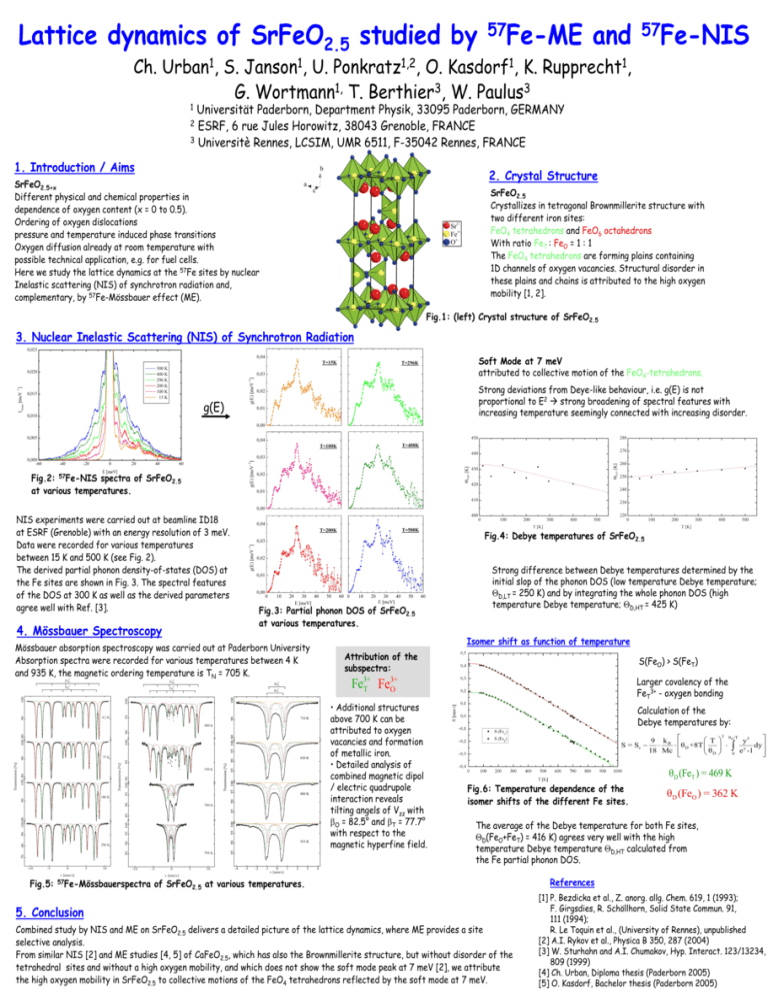
Lattice dynamics of SrFeO2.5 studied by Ch. 1 Urban , 1 Janson , 1,2 Ponkratz , 57Fe-ME 1 Kasdorf , S. U. O. K. 1, 3 3 G. Wortmann T. Berthier , W. Paulus and 57Fe-NIS 1 Rupprecht , Universität Paderborn, Department Physik, 33095 Paderborn, GERMANY 2 ESRF, 6 rue Jules Horowitz, 38043 Grenoble, FRANCE 3 Universitè Rennes, LCSIM, UMR 6511, F-35042 Rennes, FRANCE 1 1. Introduction / Aims 2. Crystal Structure SrFeO2.5+x Different physical and chemical properties in dependence of oxygen content (x = 0 to 0.5). Ordering of oxygen dislocations pressure and temperature induced phase transitions Oxygen diffusion already at room temperature with possible technical application, e.g. for fuel cells. Here we study the lattice dynamics at the 57Fe sites by nuclear Inelastic scattering (NIS) of synchrotron radiation and, complementary, by 57Fe-Mössbauer effect (ME). SrFeO2.5 Crystallizes in tetragonal Brownmillerite structure with two different iron sites: FeO4 tetrahedrons and FeO6 octahedrons With ratio FeT : FeO = 1 : 1 The FeO4 tetrahedrons are forming plains containing 1D channels of oxygen vacancies. Structural disorder in these plains and chains is attributed to the high oxygen mobility [1, 2]. Fig.1: (left) Crystal structure of SrFeO2.5 3. Nuclear Inelastic Scattering (NIS) of Synchrotron Radiation Soft Mode at 7 meV attributed to collective motion of the FeO4-tetrahedrons. Strong deviations from Deye-like behaviour, i.e. g(E) is not proportional to E2 strong broadening of spectral features with increasing temperature seemingly connected with increasing disorder. g(E) Fig.2: 57Fe-NIS spectra of SrFeO2.5 at various temperatures. NIS experiments were carried out at beamline ID18 at ESRF (Grenoble) with an energy resolution of 3 meV. Data were recorded for various temperatures between 15 K and 500 K (see Fig. 2). The derived partial phonon density-of-states (DOS) at the Fe sites are shown in Fig. 3. The spectral features of the DOS at 300 K as well as the derived parameters agree well with Ref. [3]. 4. Mössbauer Spectroscopy Fig.4: Debye temperatures of SrFeO2.5 Fig.3: Partial phonon DOS of SrFeO2.5 at various temperatures. Mössbauer absorption spectroscopy was carried out at Paderborn University Absorption spectra were recorded for various temperatures between 4 K and 935 K, the magnetic ordering temperature is TN = 705 K. Isomer shift as function of temperature Attribution of the subspectra: Fe 3+ T Fe 57Fe-Mössbauerspectra S(FeO) > S(FeT) Larger covalency of the FeT3+ - oxygen bonding 3+ O • Additional structures above 700 K can be attributed to oxygen vacancies and formation of metallic iron. • Detailed analysis of combined magnetic dipol / electric quadrupole interaction reveals tilting angels of Vzz with O = 82.5° and T = 77.7° with respect to the magnetic hyperfine field. Fig.5: Strong difference between Debye temperatures determined by the initial slop of the phonon DOS (low temperature Debye temperature; D,LT = 250 K) and by integrating the whole phonon DOS (high temperature Debye temperature; D,HT = 425 K) Calculation of the Debye temperatures by: 3 θ /T D T 9 kB y3 S = Sc θ D +8T y dy 18 Mc e -1 θD 0 θD (FeT ) = 469 K Fig.6: Temperature dependence of the isomer shifts of the different Fe sites. θD (FeO ) = 362 K The average of the Debye temperature for both Fe sites, D(FeO+FeT) = 416 K) agrees very well with the high temperature Debye temperature D,HT calculated from the Fe partial phonon DOS. of SrFeO2.5 at various temperatures. 5. Conclusion Combined study by NIS and ME on SrFeO2.5 delivers a detailed picture of the lattice dynamics, where ME provides a site selective analysis. From similar NIS [2] and ME studies [4, 5] of CaFeO2.5, which has also the Brownmillerite structure, but without disorder of the tetrahedral sites and without a high oxygen mobility, and which does not show the soft mode peak at 7 meV [2], we attribute the high oxygen mobility in SrFeO2.5 to collective motions of the FeO4 tetrahedrons reflected by the soft mode at 7 meV. References [1] P. Bezdicka et al., Z. anorg. allg. Chem. 619, 1 (1993); F. Girgsdies, R. Schöllhorn, Solid State Commun. 91, 111 (1994); R. Le Toquin et al., (University of Rennes), unpublished [2] A.I. Rykov et al., Physica B 350, 287 (2004) [3] W. Sturhahn and A.I. Chumakov, Hyp. Interact. 123/13234, 809 (1999) [4] Ch. Urban, Diploma thesis (Paderborn 2005) [5] O. Kasdorf, Bachelor thesis (Paderborn 2005)