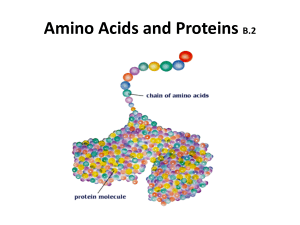
amino acids
advertisement

Once upon a time … Matthias Schleiden The Cell Theory "I must tell you that I can make urea without the use of kidneys, either man or dog. Ammonium cyanate is urea." Eduard Buchner The Nobel Prize in Chemistry 1907 Thomas Hunt Morgan was awarded the Nobel Prize in Physiology or Medicine in 1933. So, Cell Biology is a woven cloth of microscopy, biochemistry, genetics, & technology. Cell Biology lies as the foundation in a large part of our lives. Your challenge is to find it. Building the cell and making it run. Protein, their structure and function Compartments Compartment functions and intercompartment communication Electrical The Power Plant Cellular basis of tissue movement Cell to Cell signaling Cell from Cell- How and When Protein Structure and Function Enzyme: reaction catalysis, like protein degradation (pepsin), amino acid synthesis (tryptophan synthase) Structural proteins: Hair (a keratin), intracellular cytoskeleton (tubulin) Transport proteins: stomach (glucose carriers), blood stream (hemoglobin, albumin) Motors: skeletal contraction proteins (myosin) Storage: amino acids (ovalbumin), iron (ferritin) Signaling: glucose (insulin and insulin receptors) Gene regulation: DNA binding proteins (Lactose repressor) It’s all about shape. The shape of a protein is related to its function. The shape of a protein is specified by its amino acid sequence Polypeptide backbone of amino acids with side chains that specify the unique property and folding pathway. Mostly just 20 amino acids are used in polypeptide synthesis. Know the side chain type for each; not the structure. Each protein has a unique 3D structure determined by the order of the amino acids. Determining how the order of a.a. drives folding and determines the shape of a protein is the holy grail of protein chemistry. Denaturation/renaturation studies have been informative. Take home lesson: Proteins seek structures that have minimal free energy. Non-covalent forces drive the folding pathway and maintain the final 3D structure. Hydrogen bonds, ionic bonds, and van der Waal attractions The side chains drive the folding. Hydrogen bonding is the most important. While low energy, … lots of ‘em. Only a few structures out of many possibilities are stable n 20 10390 primary sequences for a 4 amino acid polypeptide 9 10 seconds if you live to 65 years Misfolded proteins can lead to neurodegenerative diseases. Mad Cow Alzheimer’s and Huntington’s …also prion diseases like BSE (cows), Scrapie (sheep), CFJ (humans) Parallel or antiparallel? Silk, antifreeze, etc Hair, hair, long, beautiful hair… skin too Protein Structure hierarchy Primary structure (sequence the protein- the first was insulin in ‘55, actually easier to sequence the gene and deduce the protein sequence.) Secondary Structure (alpha helix, beta sheet) Tertiary (jelly roll, beta barrel, zinc fingers, coiled coil) Quaternary Structure (homo-, hetero-, dimers, trimers, tetramers, etc) What is in a domain? A domain by any other domain would smell as sweet? Take for example: hemoglobin, four oxygen carrier, heterotetramer