Lecture 7 ppt
advertisement
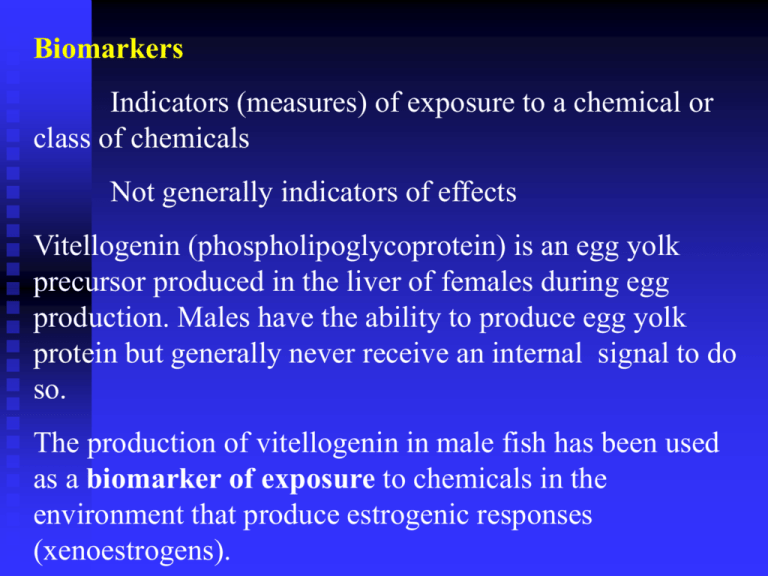
Biomarkers Indicators (measures) of exposure to a chemical or class of chemicals Not generally indicators of effects Vitellogenin (phospholipoglycoprotein) is an egg yolk precursor produced in the liver of females during egg production. Males have the ability to produce egg yolk protein but generally never receive an internal signal to do so. The production of vitellogenin in male fish has been used as a biomarker of exposure to chemicals in the environment that produce estrogenic responses (xenoestrogens). Estrogen (estradiol) and its metabolites (listed in order of their potency) estrone, and estriol have all been shown to be capable of inducing the production of vitellogenin in male fish. Ethynyl estradiol is a synthetic estrogen that is used in the Pill. It too has been shown to be capable of inducing the production of vitellogenin in male fish. Should we care that male fish have elevated levels of VTG, and if so why? Death rate < Birth rate Ova Sperm Individual Born Lives Reproduces Dies ? Population Increases Population Decreases Population Persists Total Fertility 2.1 Death rate > Birth rate Two systems control all physiological processes The nervous system exerts point-to-point control through nerves, similar to sending conventional messages by telephone (hard wired). The endocrine system broadcasts its hormonal messages to essentially all cells by secretion into blood and extracellular fluid. Like a radio broadcast it requires a receiver (cells must have a receptor for the hormone being broadcast). These two system often act together Endocrinology is the study of hormones, their receptors and the intracellular signaling pathways they invoke. Distinct endocrine organs are scattered throughout the body. Hypothalamus Pituitary Parathyroid glands Testes Thyroid glands Pancreas Adrenal glands Ovaries In addition to the classical endocrine organs, many other cells secrete hormones, through what is sometimes called the “diffuse” endocrine system. All pathophysiological events are influenced by the endocrine milieu All “large” physiologic effects are mediated by multiple hormones acting in concert There are many hormones known and little doubt that others remain to be discovered. What exactly are hormones and how are they different from “non-hormones”? Hormones are chemical messengers secreted into blood or extracellular fluid by one cell that affect the functioning of other cells. Most hormones circulate in the blood, coming into contact with essentially all cells. However, a given hormone usually affects only a limited number of cells, which are called target cells. Target cells respond to the hormones because they have receptors for the hormone. Hormone receptors are found either exposed on the surface of the cell or within the cell depending on the type of hormone. In very basic terms binding of hormone to receptor triggers a cascade of reactions within the cell that affects function. Two important terms are used to refer to molecules that bind to the hormone-binding sites of receptors: Agonists are molecules that bind the receptor and induce all the post-receptor events that lead to a biologic event. In other words they act like the “normal” hormone. Antagonists are molecules that bind the receptor and block binding of the agonist, but fail to trigger intracellular signaling events. (Antagonists are like certain types of bureaucrats – they don’t themselves perform useful work, but block the activities of those that do have the capacity to contribute.) Hormone antagonists are widely used as drugs (e.g. statins). Atorvastatin (Lipitor) Fluvastatin (Lescol) Lovastatin (Mevacor) Pravastatin (Pravachol) Simvastatin (Zocor) Rosuvastatin (Crestor) The statin drugs inhibit HMG CoA reductase in the liver and prevent the formation of cholesterol. Lipitor, followed closely by Zocor was the most frequently prescribed drug in the United States. All have the same MOA and probably act additively Normal response Timing Abnormal Response No Response Dormant receptor Receptor Blocked turned on by mimic Normal receptor over stimulated by mimic Endocrine System regulates biological processes Growth and function of reproductive system (androgen [testosterone], estrogen [estradiol], and related compounds from gonads) Control of blood sugar (pancreatic insulin) Regulation of metabolism (adrenal cortisol and thyroid thyroxin) Development of nervous system including brain (estrogen and thyroid hormones) Overall development from conception to old age = homeostasis Hormone Classes Steroids : derived from cholesterol androgens and estrogens and cortisol Amines : synthesized from amino acids give rise to adrenaline and noradrenaline Peptides and proteins : a.a. chains growth hormone Eicosanoids - 20 carbon fatty acid derivatives prostoglandins = adenyl cyclase activator, cellular energetics Receptor Types Cell membrane : peptide hormones Cytoplasmic : steroids Nuclear receptors : thyroid hormones a cell may contain as many as 10,000 receptors for a single hormone ~ 50-100 genes may be controlled by a single hormone Specific Case Example Fish Response to Wastewater Effluent Estrogenicity Estrogen Mimics Intended disruptors DES (diethylstilbestrol) ethynylestradiol phytoestrogens (soy protein, soy milk) Paper mills, Canada Estrogen Mimics – death by “progress” Unintended disruptors nonyl/octylphenol commercial and domestic detergents phthalate & Bisphenol A plastics : bottles and liners DDT (DDE) PCBs Estrogen Mimics : promiscuous receptor Estradiol Intended disruptors Ethynylestradiol DES Estrogen Mimics : promiscuous receptor Estradiol Unintended disruptors -C9H19 Bisphenol A Polycarbonate plastic DDT DEHP Di(2-ethylhexyl) phthalate Nonylphenol Vitellogenin Biomarker : fish model Endogenous / Exogenous Estrogens Vitellogenin Liver Gonad Vitellogenin – egg yolk precursor produced in the liver Pilot Study Methods Adult male fathead minnows (n=5/exposure) 1-3 Weeks of exposure to 100% WWTP RHW control in lab Plasma VTG content via ELISA Likelihood Ratio Test ND left censored at 3,000 ng/ml Pilot Study Results Number of male fish with detectable VTG levels / site with 5 fish 5 4 3 2 1 0 Control 1 Week 2 Weeks 3 Weeks Non-detects were left censored at 3,000 ng/ml = DL. Pilot Study Results Mean plasma vitellogenin concentrations (ng/ml) 30000 * 25000 * 20000 15000 10000 5000 0 Control 1 Week 2 Weeks 3 Weeks *Statistical significance from the controls (=0.05) Comparison to Similar Studies Mean plasma vitellogenin concentrations (ng/ml) in fish exposed to wastewater effluent receiving systems. 50000 45000 40000 35000 30000 25000 20000 15000 10000 5000 0 Background Exposed TX FHM UK TRT UK FLO MI FHM Conclusions Induction of vitellogenesis (biomarker) in male fish indicates the presence of estrogenic components in the wastewater effluent tested. Vitellogenin concentrations increased and were more frequent with increased exposure duration. Vitellogenin concentrations in male fish decreased when UNT and TWU were not in session. Additional Research Assessment of the efficacy of a constructed wetland to reduce or remove wastewater effluent estrogenicity using the vitellogenin biomarker in fathead minnows (Pimephales promelas Rafinesque, 1820). wetland vegetative coverage and degradation activity suspect chemical constituents estradiol and ethynylestradiol vs DEHP, DDT, Bisphenol A, Nonylphenol Wetland Exposure Design Replicate minnow traps (seven male fish per trap) 2 4 Out 3 1 In Fish Measurements Vitellogenin Content of Plasma GSI – Gonado Somatic Index testes wt/somatic (total body) wt x 100 HSI – Hepato Somatic Index liver wt/total body wt x 100 Hematocrit (%packed blood cells) Secondary Sex Characters (turbercles, fat pad, stripes) Length Weight Gonadal Differentiation Chemical Analysis Estradiol Ethynylestradiol -C9H19 Bisphenol A DDT DEHP Nonylphenol Wetland Characterization vegetation types and density depth and width of channels retention time as estimated by input flow relative to chemical constituents & fish response Fish health as measured by condition factor (K) and hematocrit values were significantly reduced at wetland sites 1 and 2. A negative trend was observed between VTG concentration and condition factor among wetland sites. A positive trend was observed between condition factor and hematocrit value. Gonadosomatic index was significantly reduced at site 1 in the wetland while the hepatosomatic index was significantly increased. Tubercle number, fatpad thickness, and stripe density were all reduced at site 1. Vitellogenin concentrations in fish from site 1 were significantly elevated compared to control and other wetland sites. The constructed wetland significantly reduced VTG levels in fish and increased measures of fish well-being. As population continues to increase and pressures on available freshwater in our area increase use of “reclaimed water” will become more and more important. One of the ways to reclaim the water may be to run Trinity River water through a constructed wet land and then reintroduce it into our reservoirs. What negatives can you see in this? Vitellogenin Gene Expression in Fathead Minnows Exposed to EE2 in a Whole Lake Dosing Experiment Greg Toth *Lazorchak, 1 JM1, Flick, R1, Lattier, D.L.1, U.S. EPA, National Exposure Research Laboratory, Cincinnati, OH Kidd, K2, Palace, V2, Evans, B2 , Blanchfield, P2, Mills, K2 , Hodge, T2,. 2 Canadian Division of Fisheries and Oceans, Freshwater Institute, Winnipeg, Manitoba, Smith, ME3, Wiechman, B3,, 3 Sobran Inc., c/o U.S. EPA, Cincinnati, OH., 3 Why look at 17a-ethynylestradiol (EE2) effective component of birth control pills potent estrogen mimic 70-80 % degraded in sewage treatment found at significant and effective concentrations downstream of municipal wastewater treatment plants K. Kidd Why Work with the Canadian DFO Fathead Endpoints Vitellogenin (spring, mid-summer, fall) V. Palace Liver, kidney and gonad development, GSI and LSI (spring & fall) B. Evans Secondary sex characteristics (mid-summer) P. Blanchfield Male reproductive behaviour (mid-summer) P. Blanchfield Nest size and egg development (mid-summer) P. Blanchfield Population size structure, growth, abundance (spring and fall) K. Mills K. Kidd Located in northwestern Ontario approximately 250 km east of Winnipeg and 50 km east-southeast of Kenora. K. Kidd Lake 260 – Ethynylestradiol (EE2) Addition Lake • 34 ha in surface area • Max. depth 14 m • Outflow into lake that is 30 times larger • Contains well-defined populations of lake trout, white sucker, fathead minnow and pearl dace • Long-term records on plankton and water quality Dosed during stratification Study Design recovery? effects on individuals & populations ethynylestradiol additions baseline data 1999 2000 2001 2002 2003 2004 reference lake data 2005 2006 Additions of EE2 to Lake 260, 2001-2003 • EE2 added 3 times a week for 5 months • 100-450 mg added/day to maintain constant concentration (4.5% loss/day) • Season mean of 6.1 (2.9) and 5.0 (1.8) ng/L in surface waters (SPE solid phase extraction and RIA measure hormone levels in blood without a bioassay) water sampling K. Kidd Mean EE2 Concentrations in Lake 260 14 12 2002 2001 EE2 (ng/L) 10 8 6 4 2 0 31 4-J 11 18 25 2-J 9-J 16 23 30 6-A 13 20 27 4-S 10 17 24 1-O 8-O -M un -Ju -Ju -Ju ul ul -Ju -Ju -Ju ug -Au -Au -Au ep -Se -Se -Se c c ay n n n l l l g g g p p p t t additions started Concentrations of EE2 in Stratified Lake 260 Epilimnion EE2 = 6.1+/- 1.3 ng/L Metalimnion 1.9 +/- 0.93 ng/L Hypolimnion 1.7 +/- 0.61 ng/L U.S. EPA MERB 2001 Objectives Evaluate exposure of indigenous male fathead minnows in Lake 260 to ethynylestradiol using vitellogenin gene expression Evaluate short-term exposure of male fathead minnows from Lake 114 deployed in Lake 260 Evaluate exposure of Cincinnati male fathead minnows to Lake 260 water at the US EPA facility in Cincinnati Evaluate exposure of Cincinnati fathead minnow fry to sediment from Lake 260 Approach - Indigenous Male Fathead Minnows Male Fathead Minnows were collected from Lake 260 after 7 weeks, 9 weeks and 12 weeks of dosing. Male minnows were collected from Lake 114 at the same timepoints. Livers were collected. RT-PCR was performed on samples and vitellogenin expression quantified relative to 18s ribosomal RNA expression. 2001 Semi-quantitative PCR Gel Based 2001 Results of Indigenous Male Fathead Minnows Vitellogenin Expression FHM Adult Livers - 17 Cycles 0.8 Pixel Density Vg/(Vg + 18S) 1 0.6 Average 0.4 + 1 StD 0.2 - 1 StD 0 -0.2 La ke 11 4 July 9 La ke 26 0 k La e 4 11 M k La e 0 26 July 25 M La ke 4 11 F L e ak 11 4 Sept La ke 26 0 EPA MERB Approach - Deployment Study Fish were collected in Lake 114 using minnow traps two days before deployment. On day of deployment males were separated from females and placed in cages in Lakes 114 and 260. Minnows were retrieved from cages on days 1, 3, 7 and 13. Livers were collected and RT-PCR performed. 2001 Results of 13-day Deployment Study Vitellogenin Expression FHM Adult Livers - 17 Cycles 1.2 0.8 Pixel Density Average + 1 SD - 1 SD 0.6 0.4 0.2 0 13 7 D D ay ay 7 0 0 La ke 26 26 ke La La ke 11 4 D ay 3 D ay 3 0 26 ke La La ke 11 4 D ay 1 ay D 0 26 ke La ke 11 4 D ay 1 -0.2 La Vg/(Vg + 18S) 1 EPA MERB Approach - Grab Samples Water was collected from Lakes 114 and 260. Samples were shipped to Cincinnati. Male Cincinnati fathead minnows were exposed to water samples for 48 hours with water renewal after 24 hours. Male fathead minnows were also exposed to 5 ng/L ethynylestradiol. RT-PCR was performed on RNA from liver samples. 2001 Comparison of Exposure of Minnows to Lakes 114 and 260 Grab Samples Vitellogenin Expression FHM Adult Livers - 17 Cycles 3 2 Avg 1.5 +1 Std 1 -1 Std 0.5 0 26 La ke E /L ng 5 ke La 0 E2 4 11 ne Li b La M S O -0.5 D Pixel Density Vg/(Vg+18S) 2.5 Vitellogenin Expression by QPCR* Study Number 1-11 (In-House ELA) FHM Adult Male Livers 0.9 Vg / 18S 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 DMSO Labline Lake 114 Treatment Group 5 ng/L EE2 Lake 260** EPA MERB Approach - Sediment Study Sediment samples were collected and shipped to Cincinnati. Sediment was combined with two volumes of water, shaken for one hour, and centrifuged. Liquid phase (elutriate) was used for exposures. Fry were exposed to elutriate for five days, with elutriate renewed daily. Fry were homogenized and RT-PCR was performed on the samples. 2001 Results of Fathead Minnow Fry Exposed to Sediment Samples Vitellogenin Expression Pixel Density Vg/(Vg + 18S) FHM Fry - 32 Cycles 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 -0.1 -0.2 H M Average + 1 St D -1 St D RW D SO M 5 ng /L 2 EE La ke 11 4 L e ak 2 60 #1 L e ak 2 60 #2 L e ak 2 60 #3 L e ak 2 60 #4 L e ak 2 60 #5 2002 Objectives Vitellogenin expression Evaluate exposure of indigenous male fathead minnows in Lake 260 to ethynylestradiol using vitellogenin gene Expression before and 3 times throughout the dosing. Evaluate exposure of Cincinnati male fathead minnows to Lake 260 water at the US EPA facility in Cincinnati Evaluate exposure of Cincinnati fathead minnow fry to sediment from Lake 260 2002 Vitellogenin expression Analyzed by quantitative real-time PCR Extrapolation to standard curve Use of reference Quantitation relative fluorescence 0.35 0.3 0.25 K. Kidd 0.2 0.15 0.1 0.05 0 1 3 5 7 9 11 13 15 17 19 21 23 25 27 29 31 33 35 37 39 cycle number K. Kidd 27 -M ay 26 19 -J 0 un 26 90 Ju l2 60 1O ct 26 0 ay 1 3Ju 14 n 11 54 Ju n 19 11 -J 4 un 21 11 -J 4 un 27 11 -S 4 ep 11 4 27 -M 02 2 44 2 44 ep 1- 442 O ct 44 2 24 -S 27 5- ay 14 -M Ju l3 5- Vg/18s Indigenous Fathead minnows: males 2002 Results 2.5 2 1.5 1 0.5 0 ay 1O ct 26 0 26 0 26 0 ay 19 -J un 27 -M 44 24 2 -S ep 44 2 1O ct 44 2 27 -M 27 -M 16 ay ,1 11 94 M ay 11 27 4 -S ep 11 4 Vg/18S Fathead minnows: females 2002 1.6 1.4 1.2 1 0.8 0.6 0.4 0.2 0 EPA Observations: 2002 fathead minnows Females in lake 260 continued to have higher vitellogenin gene expression into October, compared to Lake 114 and Lake 442 females A very impressive lack of vitellogenin gene expression in all males from all control lakes Males in lake 260 exhibited high vitellogenin gene expression after dosing, with expression remaining high at least until October 1 Histologically Male Fathead Minnow With Ovipositor Collected From Lake 260 In Summer Of 2003 (photo by C. Podemski). Vitellogenin (mg/g whole body homogenate) 0 10000 8000 6000 4000 2000 Fall 1999 Spr 2000 Summ 2000 Fall 2000 Spr 2001 Summ 2001 Fall 2001 Fall 1999 Spr 2000 Summ 2000 Fall 2000 Spr 2001 Summ 2001 Fall 2001 12000 11000 10000 9000 8000 2 Fall 1999 Spr 2000 Summ 2000 Fall 2000 Spr 2001 Summ 2001 Fall 2001 DFO Vitellogenin in Fathead Minnow Pre-EE2 Post EE2 Males 0 Lake 260 Lake 114 Lake 442 1000 Females DFO Histopath Results Lake 260 Fathead Minnow Testes – Spring 1999 2000 2001 100 mm 2002 100 mm spermatogonia spermatocytes 100% fibrosis, no tubules spermatozoa K. Kidd DFO Male Fathead Minnow Behaviour – Nest Defense Results 23 Nesting aggression chases per min (+ 1s.e.) 7 6 5 20 4 31 3 21 19 8 8 2 11 9 1 0 K. Kidd EE2 additions start n/a n/a n/a 1999 2000 2001 2002 1999 2000 2001 2002 1999 2000 2001 2002 Lake 302 Lake 260 Lake 375 Summary - Fathead Minnow •Spring 2001 - EE2 additions began - Vg gene expression induction in deployed 114 fish in 260 in 24-hrs –significant vitellogenin plasma induction after 7 weeks •Fall 2001 (4 months) –proteinaceous accumulation in kidney –liver cell size increased •Spring 2002 (12 months) –disorganized testes, immature ovaries –decreased spawning aggression, fewer & less-developed eggs –reduction in 2o sex characteristics – No fish population impacts observed •Fall 2002 (17 months) - reproductive failure, few age 0 fish •Spring 2003 (2 years) - only age 2 fish remaining –one male found, females with large ovipositors • 2006 (3yrs post additions) – Fathead population recovered Endocrine Disruption Legislation Food Quality Protection Act TOSCA – Toxic Substances Control Act FFIRA – Federal Insecticide, Fungicide, and Rodenticide Act Safe Drinking Water Act Ammend. develop a screening program, using appropriate validated test systems and other scientifically relevant information, to determine whether certain substances may have effects in humans that are similar to an effect produced by a naturally occurring estrogen, or other such endocrine effect as the Administrator may designate by August 1998 and implement by Aug 1999 (EPA extended the comment period on “Proposed Chemical Selection Approach for Initial Round of Screening, to April 1, 2003). Once the chemicals to be tested are chosen Tier 1 screening tests will be performed followed by Tier 2 testing where warranted. Tier 1 will be comprised of a battery of screening assays that would identify substances that have the potential to interact with the estrogen, androgen, or thyroid hormone systems. The purpose of the Tier 2 assays is to determine whether the substance may cause endocrine-mediated effects via or involving estrogen, androgen, or thyroid hormone systems, determine the consequences to the organism of the activities observed in Tier 1, and establish the relationship between doses of an endocrine-active substance administered in a test and the effects observed. They is little doubt that random blood samples taken from adults will contain as many as 200 chemicals that did not exist a century ago. EDSTAC Developed Endocrine Disruptor Screening and Testing Advisory Committee Composed of scientists and representatives from : EPA and other federal agencies, state agencies, industry, water providers, worker protection groups, environmental groups, environmental justice groups, public health groups and research scientists Endocrine Disruptor Definitions An exogenous agent which interferes with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body which are responsible for the maintenance or homeostasis, reproduction, development or behavior. Kavlock, 1996 = Too open ended for regulation Endocrine Disruptor Definitions An exogenous agent that changes endocrine function and causes adverse effects at the level of the organism, its progeny, and/or (sub) populations of organisms. EDSTAC 1997 also includes androgens and thyroid hormones EDSTAC Dilemma Examine estrogen, androgen, and thyroid (EAT) Evaluate endocrine disrupting properties of both chemical substances and common mixtures The universe of chemicals to be prioritized for screening and testing number >87,000 plus mixtures. Chemicals include pesticides (carriers) commodity chemicals, food additives, nutritional supplements, naturally occurring, non-steroidal estrogens phytoestrogens); soy Estimated Number of Chemicals Number of chemicals: 5,000,000 Chemicals in commerce: 80,000 Industrial chemicals: 72,000 New Chemicals: 2,000/year (1,000 in US) Pesticides: 600 (21,000 products) Food Additives: 8,700 Cosmetic ingredients: 7,500 (40,000 products) Human pharmaceuticals: 3,300 EPA estimates for the world, 1995. TOSCA – The law does not require routine testing of chemicals, and critics contend required tests only provide limited information about new chemicals. To approve a new chemical for commerce, EPA chemists compare its structure to a list of similar compounds. If no red flags go up, off to the market it goes. The EPA has 90 days to review a chemical, though approval typically comes earlier because the agency has accumulated enough chemistry data to fast track large categories of compounds. The EPA receives 108 applications on average per month from companies seeking to introduce new chemicals on the market – 32,559 since 1979. With the application comes “all available data” on production volume, use and environmental releases but not a word on toxicity unless the manufacturer happens to have some data. Since 1979 EPA has forced restrictions on just nine applications. Federal Law in place since 1979 (TOSCA) directs regulators to assess the hazards of chemicals in commerce and control those of greatest concern. NAS, GAO, Congressional Office of Technology Assessment, EPA have all concluded that TOSCA falls short of its objective. Europe in 2006 is set to switch to a chemical policy that requires chemicals to be evaluated for safety before going on the market (precautionary principle). Called REACH – Registration, Evaluation, and Authorization of Chemicals – the policy promises to revolutionize the way European regulators look at chemicals. They’re basically saying no data, no market, and the industry is up in arms about it. More Info Our Stolen Future, Colborn, Dumanoski, Myers, 1996. Generations at Risk, Reproductive Health and the Environment, Schettler, Solomon, Valenti, Huddle, 1999. http://website.lineone.net/~mwarhurst/chemicals.html http://www.tmc.tulane.edu/ecme/eehome/ http://www.epa.gov/scipoly/oscpendo/index.htm