Name: :______ Score: /65 Periodic Table Family Spectacular
advertisement
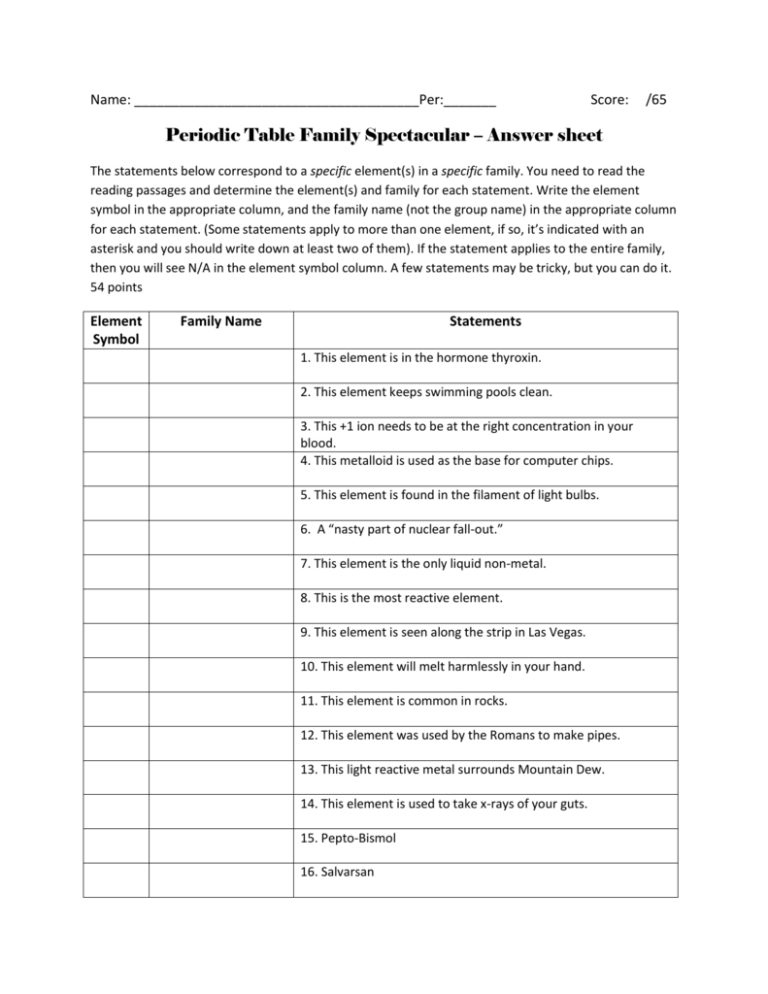
Name: ______________________________________Per:_______ Score: /65 Periodic Table Family Spectacular – Answer sheet The statements below correspond to a specific element(s) in a specific family. You need to read the reading passages and determine the element(s) and family for each statement. Write the element symbol in the appropriate column, and the family name (not the group name) in the appropriate column for each statement. (Some statements apply to more than one element, if so, it’s indicated with an asterisk and you should write down at least two of them). If the statement applies to the entire family, then you will see N/A in the element symbol column. A few statements may be tricky, but you can do it. 54 points Element Symbol Family Name Statements 1. This element is in the hormone thyroxin. 2. This element keeps swimming pools clean. 3. This +1 ion needs to be at the right concentration in your blood. 4. This metalloid is used as the base for computer chips. 5. This element is found in the filament of light bulbs. 6. A “nasty part of nuclear fall-out.” 7. This element is the only liquid non-metal. 8. This is the most reactive element. 9. This element is seen along the strip in Las Vegas. 10. This element will melt harmlessly in your hand. 11. This element is common in rocks. 12. This element was used by the Romans to make pipes. 13. This light reactive metal surrounds Mountain Dew. 14. This element is used to take x-rays of your guts. 15. Pepto-Bismol 16. Salvarsan 17. Rotten Eggs N/A 18. React violently with air or water. 19. Makes the strongest permanent magnets. * 20. Stainless steel N/A 21. The most basic hydroxides. N/A 22. Has the least compounds. 23. Has the most compounds. 24. Food cans do not rust because they are coated with this element. 25. Chain link fences do not rust because they are coated with this element. 26. Used to kill a defecting Russian spy. 27. Purple gas N/A 28. All of this group is diatomic. N/A 29. All (but one) have a stable octet of 8 electrons. 30. Liquid metal, very heavy and poisonous. * 31. Money N/A 32. The number of valence electrons varies between the elements in this family. 33. An old mine in Utah. 34. The lightest gas. 35. The heaviest gas and infamous carcinogen. 36. Best rechargeable batteries. 37. A non-toxic heavy metal. N/A 38. Best collection of flame colors. 39. Pewter 40. Volcanoes * 41. Biggest bombs * 42. Used as a structural metal. 43. A famous play makes use of this element. 44. Solid fuel for the space shuttle. 45. The Hope Diamond 46. The lightest metal 47. Was once in gasoline but now is (mostly) not. 48. To get this element, we drink milk. 49. To get this element, we eat spinach. 50. To get this element, we breathe. 51. To get this element, we go to “Jared.” 52. We are threatened with this element if we live a bad life. 53. This element lives for less than a minute. 54. One element in this group cannot form a simple positive ion. Now that you have completed the table, write down one (though there may be more) defining characteristic for each family. 11 pts. Alkali Metals: ___________________________ Alkaline Earth Metals: _________________________ Transition Metals: _______________________ Boron Family: ________________________________ Carbon Family: __________________________ Nitrogen Family: ______________________________ Oxygen Family: __________________________ Halogens: ___________________________________ Noble Gases: ____________________________ Rare Earths: _________________________________ Radioactive Rare Earths: ___________________________