CHM 101 R & P for Exam 2 2010 Big - CHM101-02
advertisement
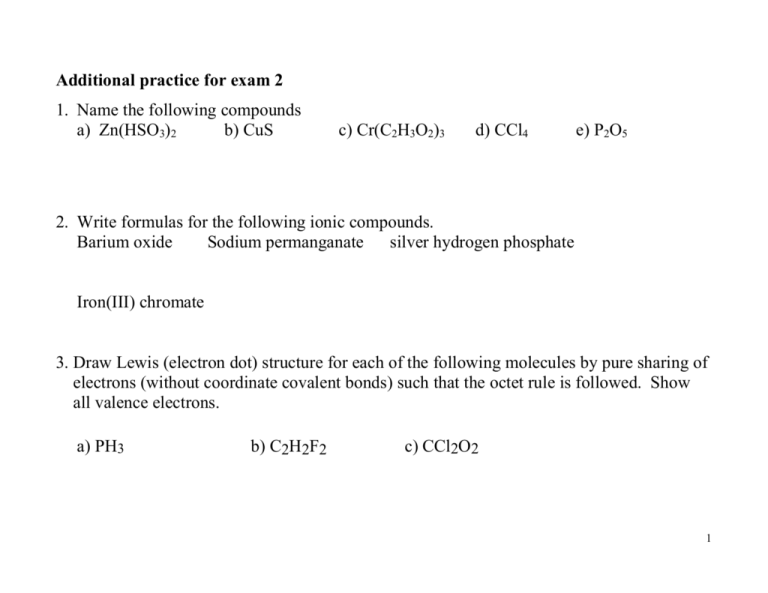
Additional practice for exam 2 1. Name the following compounds a) Zn(HSO3)2 b) CuS c) Cr(C2H3O2)3 d) CCl4 e) P2O5 2. Write formulas for the following ionic compounds. Barium oxide Sodium permanganate silver hydrogen phosphate Iron(III) chromate 3. Draw Lewis (electron dot) structure for each of the following molecules by pure sharing of electrons (without coordinate covalent bonds) such that the octet rule is followed. Show all valence electrons. a) PH3 b) C2H2F2 c) CCl2O2 1 d) CH4O e) C2H4O f) CH3NO 4. Draw the Lewis structure for each of the following ionic compounds. Show all valence electrons. a) BaF2 b) CaS c) Li2O d) Al2O3 2 5. Draw the Lewis structure for each of the following polyatomic ions. 2a) SO3 b) NO3 6. Write Lewis structures for the ionic compounds Na2SO3 and Ca(NO3)2 (see problem 5 for the negative ions). 3 7. How many ions are there in one formula unit of each of the following? a) KNO3 b) Fe3(PO3)2 c) K3P 8. Which of the following compounds is ionic? A) PH3 B) NO C) AgNO3 D) C2H6 E) SO2 9. Which of the following elements has the highest electronegativity? A) Ga B) Al C) S D) As E) P 10.How many CO2 molecules are there in 0.25 mol of CO2? 11.What is the mass in 0.25 mol of CO2? 4 12.How many moles are there in 447 g of (NH4)3PO4? 13. How many H atoms are there in question 12? 14. Which of the following has the largest number of atoms? A) 1 g of He B) 1 g of Ne C) 1 g of Ar D) 1 g of Kr 15. Which of the following is isoelectronic with Kr A) K+ B) Ca2+ C) BrD) S25 16. Which of the following bonds is most polar? A) S-P B) S-O C) N-P D) P-O E) F-F 17. For each of the following species, predict the electron pair arrangement, molecular geometry and the kind of hybrid orbital involved in the bonding of the central atom. Indicate whether it is polar or nonpolar Electron pair Molecular Hybrid Polar or arrangement Geometry nonpolar S C S 6 H S H H N H H O S O Ans. 1.a)Zinc hydrogen sulfite ; b) copper (II) sulfide; c) Chromium (III) acetate d) carbon tetrachloride e) diphosphorus pentaoxide 2. BaO, NaMnO4, Ag2HPO4, Fe2(CrO4)3 7. a)2 b)5 c)4; 8. C; 9. C; 10. 1.5 1023, 11. 11, 12. 3.00 mol , 13. 2.17 x 1025, 14. A, 15. C, 16. D 7 More practice for exam 2 1. If X is an element in group 3A and Y is an element in group 6A, what is the formula of the compound formed from X and Y? 2. How are sp3 hybrids formed? 3. Which of the following are isoelectronic? Na+, O2-, K+, F-, Sr2+ 4. What is the mass of H in 32 g of CH4? 5. How many C atoms are there in 20 g C3H8O? 8 6. Determine the total number of ions in one formula unit of each of the following. a) K2CrO4 b) (NH4)3PO4 c) Cu3P2 7. Draw Lewis (electron dot) structure for each of the following molecules by pure sharing of electrons (without coordinate covalent bonds) such that the octet rule is followed. Show all valence electrons. C4H4O N2H2 C2H5NO 8. Draw Lewis structure for each of the following ionic compounds. K2O Ca(ClO3)2 9 Answers: 1. X2Y3 3. Na+, O2-, F- 4. 8g 5. 6.0 x 1023 6. a) 3, b) 4 c) 5 10







![unit_3_part_2_prezi_pp[1]](http://s3.studylib.net/store/data/009491974_1-00bf7ba85f40f2a9d20b42cbbebf0ab5-300x300.png)