File - Mr. Lavoie's Science Webpage
advertisement

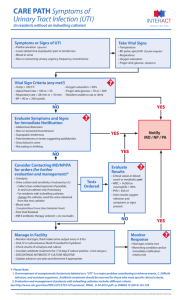
Urinalysis Veterinary Assistant Program Middlesex Community College Michael Lavoie 12/17/12 Body Tube Ocular lens (Eyepiece) Nosepiece Arm Objectives Stage Stage Clips Coarse Adjustment Diaphragm Light Fine Adjustment Base Always carry a microscope with one hand holding the arm and one hand under the base. What’s my power? To calculate the power of magnification, multiply the power of the ocular lens by the power of the objective. What are the powers of magnification for each of the objectives we have on our microscopes? Fill in the table on your worksheet. Comparing Powers of Magnification We can see better details with higher the powers of magnification, but we cannot see as much of the image. Which of these images would be viewed at a higher power of magnification? How to make a wet-mount slide … 1 – Get a clean slide and coverslip from your teacher. 2 – Place ONE drop of water in the middle of the slide. Don’t use too much or the water will run off the edge and make a mess! 3 – Place the edge of the cover slip on one side of the water drop. 4 - Slowly lower the cover slip on top of the drop. Cover Slip Lower slowly 5 – Place the slide on the stage and view it first with the red-banded objective. Once you see the image, you can rotate the nosepiece to view the slide with the different objectives. Always carry with 2 hands Only use lens paper for cleaning Do not force knobs Always store covered Keep objects clear of desk and cords Place the Slide on the Microscope Use Stage Clips Click Nosepiece to the lowest (shortest) setting Look into the Eyepiece Use the Coarse Focus Follow steps to focus using low power Click the nosepiece to the longest objective Do NOT use the Coarse Focusing Knob Use the Fine Focus Knob to bring the slide Visual Urine Attributes Volume color turbidity It is ideal to standardize the volume of urine from which the urine sediment is prepared. ◦ In human medicine, 10 ml of urine is used as the standard volume. This is difficult to accomplish in many animals, particularly small patients, hence we try and standardize the urine volume from which the urinalysis is performed (regardless of the volume received) to 3 ml Urine volume affects the results of the urine sediment examination, because the semi-quantitative results of the sediment are derived from the standard urine volume and will differ between urine collections of different volumes. Observations of color and turbidity are made on the well-mixed urine specimen. Specific Gravity This is a measure of the urine concentrating ability of the animal. The specific gravity should be read on the refractometer using the urine supernatant. Dip Stick Anaylsis Dipsticks consist of various pads containing chemical ingredients which provide a color change when a particular analyte is present in urine. This color change is converted to a semiquantitative result for the analyte in question. In animals, the dipstick is used to give results for pH, protein, glucose, ketones, bilirubin and proteins containing a heme group (blood) There are also dipstick pads for urine specific gravity, nitrate, leukocytes and urobilinogen on commercially available dipsticks. ◦ These are either not accurate in animals or do not provide much additional information in animals and are seldom reported. Sediment Examination For this examination, the standard volume of urine is centrifuged in a low speed centrifuge. The supernatant is removed and the urine is gently resuspended in a standard volume (0.5 ml) of urine supernatant. A drop of the resuspended urine is placed on a slide, coverslipped and examined under a light microscope using the 10x and 40x objectives. This looks for crystals, bacteria, blood etc. To examine do the following: Low Magnification Examine the entire coverslip using the 10x objective. ◦ At this magnification, casts, large crystals, debris, parasitic ova are semi-quantified. High Magnification Specific structures identified at low magnification (e.g. casts) and several random fields are examined using the 40x (high dry) objective. At this higher magnification, leukocytes, erythrocytes, epithelial cells, fat droplets, small crystals, sperm, debris and bacteria are semi-quantified. for information on the performance and meaning of the test. Indications for Urine Collection Diagnostic, (urinalysis, radiographic procedures), Therapeutic (urethral blockage, inability to urinate) Methods of Collecting Voided Samples Voided: Easiest method, no special knowledge or special skill needed. 1) Technique: The urine caught during normal urination in mid-stream in a clean or sterile container. 2) Problems: These samples are considered non-sterile and are often contaminated with bacteria and cells from the lower urinary tract. ◦ If this method is used for an UA (urine analysis) it should be noted on lab report. Manual Expression of the Bladder This method is often used in patients unable to urinate such as neurological and spinal patients. 1) Technique: The patient can be standing, or in lateral recumbency. ◦ One person might need to restrain patient and another express the bladder depending upon the patient. ◦ Locate bladder via palpation and apply gentle, steady pressure with hand(s) to push urine out of bladder. ◦ With steady pressure resistance and inhibitory reflexes of sphincter muscles should be overcome. Complications 2) Possible problems: Trauma to or rupture of bladder This method also produces a "contaminated" sample, usually unsuitable for an accurate UA. Catheritization 1) General information: a) This method can be used in both sexes and most species, but is more difficult in females and as the patient gets smaller. b) It is essential to maintain sterility and avoid iatrogenic infection. c) Catheters should be lubricated before insertion with sterile gel. d) Long hair in the urethral area can be clipped and the urethral area is cleaned with sterile water to avoid contamination e) The correct size catheter is important to avoid kinking, urine leakage (too small) or causing trauma to the urethra (too large). f) Pre-measure (estimate) the distance to the bladder. A catheter inserted too far into the bladder can cause trauma and hematuria or even turn on itself and become blocked, or tie itself into a knot, which makes it REALLY hard to remove! Male Dog Catheterization Instruments: ◦ Sterile plastic semi-flexible catheter or soft red rubber feeding tube and syringe or container to collect urine. If the catheter is too flexible or too small it can be difficult to place. ◦ Catheter size is expressed in "French" units (Fr), the smaller number indicates a smaller diameter catheter. Technique Two or more people may be needed depending on patient, usually done in standing or lateral position (unless anesthetized). The assistant exteriorizes the penis and it is gently cleansed with sterile water or very mild disinfectant. The catheter is handled in a sterile manner (with gloved hands or using the finger tab technique - to be demonstrated in lab). The catheter tip is lubricated with sterile jelly. The catheter tip is inserted into the penile orifice and is threaded up the urethra into the bladder. The catheter may be blocked or meet resistance at 2-3 points. If this happens rotate it and increase pressure slightly. Possible problems :Trauma to urethra or lining of the bladder. Infection if nonsterile catheter is used. Indwelling Catheters Depending upon the situation the catheter may be sutured in place for a period of time to allow the constant drainage of urine and prevent re-blockage. ◦ Usually a UCS (Urinary Collection System) is used These cats should be maintained on a grate, as they will rapidly soil blankets or towels in their cages. In cats do not allow them to use a litter pan, and litter can work its way up the catheter into the bladder. Female Dog Catheterization It is much more difficult to catheterize females because of the internal position of the urethra, often cystocentesis is the preferred method of collection of a sterile sample for this reason. a) Equipment: Rigid metal catheter, semi-rigid plastic catheter, Foley catheter, vaginal or nasal speculum, otoscope, etc. Techniques The bitch is restrained in the standing position if not under anesthesia, or the catheter can be placed in V/D or D/V position if under anesthesia. The vulva is cleansed with sterile solution or mild disinfectant and the sterile catheter is handled with gloved hands. There are several placement techniques: ◦ Visual: A speculum (lubricated with sterile gel) with a light source is used to dilate the vagina and locate the urethral orifice on the ventral surface of the vagina. Restraint for urine collection and collection http://www.youtube.com/watch?v=HHFlU 0Ry_tY&feature=player_detailpage http://www.youtube.com/watch?v=mrmE QxUh4YA&feature=player_detailpage http://www.youtube.com/watch?v=HqRPS XgjaAE&feature=player_detailpage http://www.youtube.com/watch?feature=p layer_detailpage&v=ht150ZFT0Ss Cow Urine Collection http://www.youtube.com/watch?feature=p layer_detailpage&v=lJ__wNhbWn0 Bladder Expression http://www.youtube.com/watch?feature=p layer_detailpage&v=9_9PtTP3PPY http://www.youtube.com/watch?v=yoYWs 6OEfJI&feature=player_detailpage Urinary Sediments Formed elements: epithelia, red cells, white cells Crystals Mucus Renal casts Microorganisms Sternheimer-Malbin stain Microscopic UA Correlate with cloudiness and other findings Quality control ◦ ◦ ◦ ◦ ◦ Consistent volume Centrifugation Well mixed fresh specimen Microscopy (wet mount, use low light) Sternheimer-Malbin stain Epithelia Squamous epithelia ◦ Large flat cell with central oval nucleus Transitional (bladder) epithelia ◦ Spindle shaped with large oval nucleus ◦ Maybe in sheet Renal tubular epithelia ◦ Small cell with large oval nucleus ◦ Most clinically significant Squamous Epithelia Transitional Epithelia Renal Tubular Epithelia Leukocytes Pus, or pyuria May indicate urinary tract infection UTI if more than 10/HPF Glitter cells in dilute alkaline urine Pyuria Bacteriuria Erythrocytes Hematuria may indicate renal damage Menstrual contamination May be crenated or ghost cells Hematuria Renal Casts Cylindruria ◦ ◦ ◦ ◦ Renal stasis Acidic pH Proteinuria Concentrated urine Tamm-Horsfall mucoprotein matrix Renal Casts Hyaline ◦ Least significant ◦ Not refractile Coarse and fine granular Cellular ◦ Classified by cells in the cast Waxy ◦ End stage renal disease Hyaline Cast Fine Granular Casts Coarse Granular Cast RBC Casts WBC Casts Epithelial Casts Fatty Cast Broad Cast Waxy Casts Mucus Usually of no clinical significance Common Crystals in Acid pH Amorphous urate ◦ Orange powder ◦ May clear with warming or saline Uric acid ◦ Brown lemon shaped or star shaped ◦ Birefringent with polarized light Calcium oxalate ◦ Envelope Amorphous Urate Uric Acid Calcium Oxalate Common Crystals in Alkaline pH Amorphous phosphate ◦ White powder ◦ May clear with saline Triple phosphate ◦ Coffin lid Ammonium biurate ◦ Thorn apple Calcium carbonate ◦ Effervesce with SSA Amorphous Phosphate Triple Phosphate Ammonium Biurate Calcium Carbonate QUESTIONS?