Thermodynamics
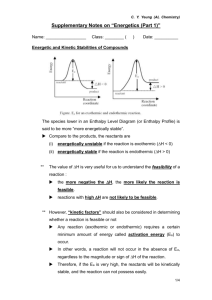
advertisement

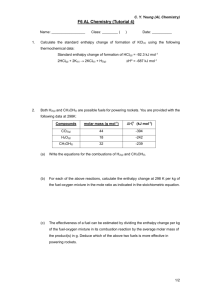
Thermodynamics Modern Methods in Heterogeneous Catalysis F.C. Jentoft, November 1, 2002 Outline Part I: Reaction + Catalyst 1. Thermodynamics of the target reaction 2. Thermodynamics of catalyst: bulk (see classes on solids and defects) and surface 3. Thermodynamics of interaction between reactant and catalyst (see class on adsorption) Part II: Practical Matters 1. Vapor pressure What Thermodynamics Will Deliver… Gives “big picture”, essence, useful for estimates Target Reaction - Motivation Why look at TD? …can’t change it anyway by catalysis E without catalyst E with catalyst EA EA Reactants Reactants Products Reaction coordinate Products Reaction coordinate Target Reaction – Quantities to Look at Enthalpy of reaction ΔrH exothermic / endothermic ΔrH of side reactions Free Enthalpy (Gibbs Energy) ΔrG exergonic / endergonic Equilibrium Constant K: Equilibrium Limitations Change of Temperature and Pressure (variables) Enthalpy of Reaction Determines reactor setup (see classes on catalyst testing and reaction engineering) catalyst formulation / dilution “hot spots” / heating power isothermal operation in the lab Enthalpy of side reactions parallel / secondary reactions Enthalpy of Reaction, ΔrH Reaction enthalpy needs a reaction equation!!! A A B B CC D D Calculate from enthalpies of formation of products and reactants L r H i f H i i 1 ΔrH°: ΔfH°: vi: standard enthalpy of reaction standard enthalpies of formation stoichiometric factors, positive for products, negative for reactants Things to Watch in Calculations….. Stoichiometric factors Standard conditions State of the matter (solid, liquid, gaseous) Which data are available (sometimes only enthalpy of combustion, ΔcH° ) Standard Conditions (IUPAC) International Union of Pure and Applied Chemistry (IUPAC) www.iupac.org Größen, Einheiten und Symbole in der Physikalischen Chemie VCH , Weinheim 1996 FHI library 50 E 49 (English version: 50 E 48) Standard state indicated by superscript ,° Standard Conditions (IUPAC) „Standard state pressure“(IUPAC 1982) p° = 105 Pa „Standard atmosphere“ (before 1982) p° = 101 325 Pa = 1 atm „Standard concentration“ c° = 1 mol dm-3 „Standard molality“ m° = 1 mol kg-1 „Standard temperature“ ?? Standard Conditions (Textbooks) Atkins STP „Standard temperature and pressure““ p = 101 325 Pa = 1 atm, T° = 273,15 K SATP „Standard ambient temperature and pressure“ p° = 105 Pa = 1 bar, T° = 298,15 K Wedler „Standarddruck“ p = 1.013 bar = 1 atm = 101.325 kPa „Standardtemperatur“ T° = 298,15 K Standard Conditions (Other) Catalysis Literature NTP „Normal temperature and pressure““ 20°C and 760 torr 70 degrees F and 14.7 psia (1 atmosphere) Sources for Thermodynamic Data CRC Handbook of Thermophysical and Thermochemical Data Eds. David R. Lide, Henry V. Kehiaian CRC Press Boca Raton New York 1994 FHI library 50 E 55 D'Ans Lax Taschenbuch für Chemiker und Physiker Ed. C. Synowietz Springer Verlag 1983 FHI library 50 E 54 Some Examples: Combustion Combustion of hydrogen (Knallgasreaktion) 1 O2 ( g ) H 2 ( g ) H 2O( g ) 2 ΔcH° = -286 kJ mol-1 Combustion of carbon C(s) O2 ( g ) CO2 ( g ) ΔcH° = -394 kJ mol-1 Reactions with CO2, H2O or other very stable molecules as products are usually strongly exothermic, however…. Steam Reforming of Methanol CH3OH ( g ) H 2O( g ) CO2 ( g ) 3H 2 ( g ) ΔcH° = 93 kJ mol-1 State of the Matter Formation of benzene at 298.15 K 6 C ( s) 3 H 2 ( g ) C6 H 6 ( g ) ΔfH° = 82.93 kJ mol-1 6 C ( s) 3 H 2 ( g ) C6 H 6 (l ) ΔfH° = 49.0 kJ mol-1 Enthalpy of evaporation of benzene? ΔvapH° = 30.8 kJ mol-1 at 80°C Partial Oxidation of Propene Oxidation of propene to acrolein 1 C3 H 6 O2 C3 H 4O H 2O 2 ΔrH° = ??? kJ mol-1 Examples for Sources Examples for Sources Partial Oxidation Only enthalpy of combustion, ΔcH°, of acrolein is given C3 H 4O( g ) 3.5O2 ( g ) 3CO2 ( g ) 2H 2O( g ) ΔcH° = -1633 kJ mol-1 Enthalpies of combustion are easily determined quantities (e.g. from quantitative combustion in a bomb calorimeter) Use Hess’s Law 3C( s) 2H2 ( g ) 4 O2 ( g ) 3CO2 ( g ) 2H2O( g ) ΔcH° = -1754 kJ mol-1 C3H 4O(l ) 3.5O2 ( g ) 3CO2 ( g ) 2 H 2O( g ) ΔcH° = -1633 kJ mol-1 3C ( s) 2 H 2 ( g ) 0.5O2 ( g ) C3H 4O(l ) Enthalpy is a State Function ΔfH° = -121 kJ mol-1 Partial vs. Total Oxidation Oxidation of propene to acrolein 1 C3 H 6 O2 C3 H 4O H 2O 2 ΔrH° = -427 kJ mol-1 E EA Reactants Oxidation of acrolein to CO2 EA Partial Oxidation Product Total Oxidation Products Reaction coordinate C3 H 4O( g ) 3.5O2 ( g ) 3CO2 ( g ) 2H 2O( g ) ΔcH° = -1633 kJ mol-1 Dehydrogenation vs. Oxidative Dehydrogenation Dehydrogenation of isobutane to isobutene i C4 H10 ( g ) i C4 H 8 ( g ) H 2 ( g ) ΔrH° = 117 kJ mol-1 Oxidative dehydrogenation of isobutane to isobutene i C4 H10 ( g ) 0.5O2 ( g ) i C4 H 8 ( g ) H 2O( g ) ΔrH° = -124 kJ mol-1 Oxidative Dehydrogenation: Thermodynamic Traps Combustion of isobutene i C4 H8 ( g ) 6 O2 ( g ) 4 CO2 ( g ) 4 H 2O( g ) ΔcH° = - 2525 kJ mol-1 Nevertheless, the oxidative dehydrogenation of isobutene is in commercial operation (CrO3/Al2O3 or supported Pt catalyst) Dehydrogenation Dehydrogenation of ethylbenzene to styrene C8 H10 (l ) C8 H 8 (l ) H 2 ( g ) ΔrH° = 117 kJ mol-1 Change of ΔrH with Temperature Most of the time, we are not interested in room temperature Enthalpy Products, T2 Reactants, T2 ΔrH1 Δ rH 2 Products, T1 Reactants, T1 Reaction coordinate How to Calculate ΔrH as Function of T Each enthalpy in the reaction equation changes according to Kirchhoff’s law TE H 2 H1 dH H1 C p dT TA And, if Cp = constant over the temperature range of interest TE dH C p dT C p T TA T2 r H T2 r H T1 C p dT T1 Heat Capacity as a Function of T, Condensed Phases Heat Capacity as a Function of T, Gases How to Calculate ΔrH as Function of T Cp as a function of temperature is usually a polynomial expression such as T1 T2 C p C a b 2 ... K K If there is a phase transition within the temperature range, it must be accounted for TU TE TA TU dH C p1dT U H C p 2 dT Isomerization Isomerization of butane n C4 H10 ( g ) i C4 H10 ( g ) ΔrH° = - 7 kJ mol-1 ΔrS° = -15 J mol-1 ΔrG°= - 2.3 kJ mol-1 Consistency check.... G H TS Free Enthalpy ΔrG, and Equilibrium Constant K Composition dependence of ΔrG L r G r G RT ln ai i i 1 Thermodynamic equilibrium constant K th ai i (dimensionless) i Relation between ΔrG° and K in equilibrium, ΔrG=0 r G RT ln Kth Different Equilibrium Constants K Kp K p pi i [Pai] i correlation between Kth and Kp K th po i L pi i i L i fi i For low pressures (a few bars and less), the fugacity coefficients are about 1 All pressures, including po should be in the same units. Kth po i Kp Isomerization Equilibrium Isomerization of butane ΔrG°= - 2.3 kJ mol-1 Kth e G RT 2.53 With Kth po i K p and K p p vi K x n C4 H10 ( g ) i C4 H10 ( g ) 28 % 72 % at 298 K Equilibrium Constant Temperature Dependence H ln K 2 T RT p ln K p H const . RT K p ,T 2 H 1 1 T T K R p , T 1 2 1 p van’t Hoff’s Equation Indefinite integration Definite integration Equilibrium Temperature Dependence 100 90 80 n -Butane 70 60 50 40 30 Isobutane 20 10 0 200 250 300 350 400 450 500 550 600 650 700 Temperature / K H= f(T); Cp = const. Fraction % Fraction % H = const. 100 90 80 n -Butane 70 60 50 40 30 Isobutane 20 10 0 200 250 300 350 400 450 500 550 600 650 700 Temperature / K Start your research by calculating the thermodynamics of your reaction! Part II: Practical Matters Vapor pressure and saturators Gas in Gas out Saturator, 100 ml Methanol 79.17 g, is 2.47 mol Methanol Thermodynamic Data Heat Consumed by Evaporation Assumption: saturator is adiabatic, evaporate 20 ml of methanol, all energy for evaporation taken from remaining 80 ml methanol 20 ml is about 0.5 mol, need about 17.7 kJ for evaporation 80 ml is about 2 mol, Cp of liquid MeOH is 81.6 J mol-1 K-1 The temperature of the methanol would theoretically drop by 108 K The Clausius-Clapeyron Equation S H p T coex. V TV General differential form of the Clausius-Clapeyron Equation H p p 2 T RT coex. For sublimation and evaporation assumes ideal behavior of the gas phase H ln pT1 R pT2 1 1 T1 T2 August’s vapor pressure formula assumes enthalpy is constant within given temperature range Vapor Pressure and Temperature At 64.4°C, the vapor pressure of methanol is 755 torr and the enthalpy of evaporation is 35.4 kJ mol-1 T1 = 337.6 K, p = 100.66 kPa pT2 pT1 e H 1 1 R T1 T2 The carrier gas will dissolve in the liquid and the vapor pressure will be lowered Methanol Vapor Pressure H assumed constant 30 300 25 Vapor Pressure / kPa 350 250 200 150 100 15 10 Temperature / K Small temperature changes can cause significant changes in vapor pressure 3 30 1 30 9 29 7 29 5 29 3 29 1 29 9 28 7 28 28 0 36 0 35 0 34 0 33 0 32 0 31 0 0 29 30 Temperature / K 5 0 0 0 20 5 50 28 Vapor Pressure / kPa H assumed constant