Chemical Bonding and Lewis Structure Class Work For all
advertisement

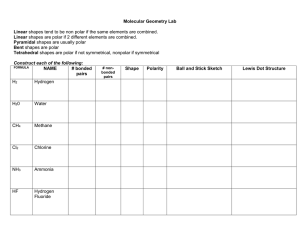
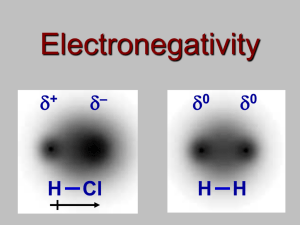
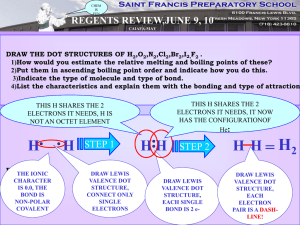
Chemical Bonding and Lewis Structure Class Work For all compound formulas given draw the Lewis Structure, identify the VSEPR shape, and indicate the bond angle: CH3I ONF SeH4 CO3-2 PF5 SiCl4 NO3-1 ClF2 OF2 PCl3 CNS-1 SF6 1) What is polarity? (Provide an example) 2) Identify the polarity of: Br – O 3) Determine the type of bond Li – O 4) Which of the two bonds are more polar? S – P or B–N 5) Describe electronegativity as best you can in terms of the atom itself. 6) Identify the type of bond: a. N – O b. K – I 7) Which of the bonds in question 6 is more polar? 8) Draw the symbol that indicates polarity and explain its meaning. For questions 1-5 draw the polarity, Identify the type of each bond, and circle the more polar of the two polar covalent molecules: 1. . C-O and As-O 2. . H-F and H-Cl 3. . B-O and C-S 4. . Pb-O and Al-O 5. . Si-Br and P-Br