Eliza-GEOS lesson plan- week 2
advertisement

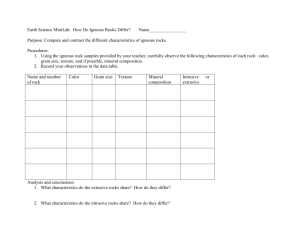
Eliza Marks Week 2 Lesson Plans Summerbridge: Geoscience During the second week students will learn about minerals, their composition, and how to classify these minerals. Students will also be introduced to the three types of rock, and look in detail into Igneous Rocks, how they are formed, and how to classify them. In this week we will also examine different types of Volcanoes. MONDAY June 25: intro to chemistry and isotopes MATERIALS Smart board YouTube Classroom handout 3 sets of isotope matching cards (make) homework sheet: half lives end-slip with one half-life problem OBJECTIVES Students will identify the different types of an atom. Students will apply the idea of carbon dating to calculating half life. PROCEDURE 1. warm-up a. pass handout out to students: intro to atoms. Have them read it over and fill in blanks if they know they b. show them intro to atoms video (1:30) http://www.youtube.com/watch?v=2gr9-7TaIiQ c. have look at sheet again and try to fill in answers in pairs d. show video one more time 2. go over the atom classroom worksheet as a class a. go around the classroom and each student fills in one of the blanks. I will write the answers on the board b. we will go over the blanks again using a powerpoint, and add diagrams to the handout in the spaces below 3. isotope a. definition: an isotope is an element that has the same atomic number (number of protons), but a different atomic mass (number protons + neutrons because protons and neutrons are each one AMU) b. ACTIVITY: put 10 cards on each table and have students match the isotopes c. Come together and give answers of isotopes (each card has a letter so we can put the letters in groups) 4. How can we date rocks and fossils? a. Carbon dating i. Read to class: “Radiocarbon Dating One method that scientists use to date ancient fossils and artifacts is called radiocarbon dating. All living things on Earth are made up of a high percentage of an element called carbon. Carbon combines with other elements in complex ways to form the molecules that make up our bodies. Most carbon on Earth is not radioactive, but a very small percentage is. Thus, as living things take in carbon, they inevitably will take up a small amount of radioactive carbon into their bodies. When these life forms die, they stop taking in new carbon. The carbon in their bodies at the time of their death will remain in their bodies until they decompose, or if they become fossilized, then forever. Radioactive carbon decays at a known rate. This allows scientists to look at the amount of decay in a fossil’s radioactive carbon and determine a relative date. ii. WRITE Definition: the determination of the age of an organic object from the relative proportions of the carbon isotopes carbon-12 and carbon-14. Look at the content of the radioisotope C-14 iii. Break down definition iv. All things on earth are made up of a high percentage of carbon. v. We can use the ratio of carbon-12 and carbon-14 to find out how old something is using half life (half life is vi. n = number of half-lives, 1. percent of original remaining= 100/(2n) vii. If an element’s half life is 50 years, how long does it take for the sample to decay 50%? 50 years viii. If an element’s half life is 25 years, how long does it take for the original sample to decay 75%? 50 years ix. If 40 percent of the original sample remains, how many half lives have passed? 5. cool down … putting two and two together a. how can we use carbon dating to tell us the age of rocks/fossils b. THUMBS UP/DOWN who understood… JAPAN: GESCIENCE HOMEWORK SHEET DUE TUESDAY JUNE 26 CARBON DATING WORKSHEET 1. You have an isotope with a half-life of 200 years. How much of the original material is left after 600 years? (answer with a fraction or percent) 2. 25% of the original material is left after 1000 years. What is the material’s half-life? 3. When we do radioactive carbon dating, what carbon isotopes are we considering? 4. Draw an atom, and label the nucleus, neutron, proton, and electron cloud. TUESDAY June 26: Minerals MATERIALS Paper and pencils Chalk board Smart board soap paper clips OBJECTIVES Students will classify minerals using observations they made. Students will distinguish between minerals and non-minerals, applying characteristics of bonds they learned yesterday. Students will articulate why it is important to identify minerals in groups of 2 or 3. PROCEDURE 1. warm-up (10 minutes) a. ANSWER half-life question on the board: i. If 12.5% of the original (“parent”) material is left after 1,200 years, what is the material’s half-life ii. Write up the equation triangle iii. Identify what we are given, write down equation, substitute 2. introduction to minerals (7 minutes) a. write on board and then pass out note-sheet b. what do you know about minerals? All minerals are: i. Naturally occurring ii. Inorganic (never living) iii. Crystalline solid: orderly repeating patterns of molecules in 3D iv. Specific chemical composition 1. Each molecule has a specific combination of elements, which indicate which mineral it is c. how can we classify minerals? i. On a atomic level: Using chemical composition and physical structure…what pattern do the molecules form, and how do they combine 1. some structures: isolated structure, chains, sheets a. isolated structure b. chains: single chain or double c. sheets: bonding on planes ii. Without a machine: use color, texture, density, smudge, hardness, scent, taste, acid d. how can you make a perfect crystal (put picture on board) i. in an uninterrupted environment cave or cavity 3. OBSERVATION ACTIVITY: (20 minutes) a. Pass out an unknown material (bar of soap), paper clips, and wax paper out to each table b. Define the physical properties we can use to classify minerals (at the bottom of the hand out c. Have each table fill out the observations sheet, making sure they out at least one observation in every column d. We will go around the room and fill out an observation table on the board 4. Apply observation techniques to minerals a. Put photos of Halite, calcite, sulfur, and graphite on the board b. Have students write down observations about each mineral using the physical properties sheet, and then we can talk about them as a class c. We will write traits on the board and then I will add distinguishing traits they cannot deduce from the photo i. Halite: “salt”, we cannot be scratched by a fingernail, has cubic crystals, salty taste ii. Calcite: reacts with HCL, rhombic crystals iii. Sulfur: rotten egg smell when under heat iv. Graphite: “pencil lead”, soft metallic mineral, gray streak Resource: http://facweb.bhc.edu/academics/science/harwoodr/geol101/labs/minerals/ 5. cool down: (5 minutes) a. why is it important to be able to identify minerals? i. They tell us about the environment in which they were created ii. Minerals make up rocks…rocks are a combination of minerals. They can tell us the characteristics of rocks (sometimes we can see the crystals or minerals in a rock) JAPAN: GESCIENCE CLASSROOM HANDOUT TUESDAY JUNE 26 MINERALS Name: _______________________________________________________________ Every minerals is: 1. Naturally occurring a. Humans cannot make minerals in a lab 2. Inorganic a. Minerals were never living 3. Crystalline solids a. Minerals are solid materials, whose atoms, molecules, or ions are arranged in an orderly, repeating pattern 4. Specific chemical composition a. Minerals are composed of only one specific molecule or combination of molecules What physical properties can we use to classify minerals: 1. Color: a. What color is the sample? Is it multiple colors? Does it look like a material you see in everyday life? 2. Texture: a. Examine the sample. Can we see crystals? How big are they? What shape are they? 3. Magnetism: a. Is the sample attracted to a magnet? 4. Hardness: a. Can we scratch the sample with a sharp object? Does the sample scratch glass? b. We measure hardness using a scale 5. Streak: a. Does the sample make a colored streak when scraped across a piece of porcelain? 6. Taste: a. Does the samples have a specific taste? 7. Reaction to HCl: a. Does the samples will fizz if you put a drop of the Hydrochloric Acid on it 8. Luster: a. Does the sample reflects light? 9. Scent: a. Does the sample smell like anything? UNKNOWN MATERIAL CLASSIFICATION CLASSROOM HANDOUT TUESDAY JUNE 26 COLOR : TEXTURE : MAGNETISM (YES/NO) : HARDNESS : STREAK: TASTE (DON’T ACTUALLY TASTE, GUESS): LUSTER : SCENT: OTHER: JAPAN: GESCIENCE HOMEWORK SHEET DUE WEDNESDAY JUNE 27 MINERALS POEM NAME _____________________________________________________________________________________ Write a poem about what we learned about minerals. MINIMUM: 10 lines INCLUDE: - 2-4 lines: What 4 characteristics used to classify a substance as a mineral - 1 or more lines: Where minerals form (if crystals) - 2 lines or more: using observations about one of the minerals discussed today - 2 lines or more: use observations about another mineral we discussed today WEDNESDAY June 27: Introduction to rock formation and igneous rocks MATERIALS Sticker Igneous rock card games Chalkboard smartboard Photos GRANITE AND BASALT OBJECTIVES Students will identify the three types of rock by how they are formed (sedimentary, metamorphic, and igneous). Students will differentiate between various rocks considering texture and chemical composition, and use their conclusions to observe rocks. Students will deduce why the grain size of igneous rocks varies, considering cooling speeds. PROCEDURE 1. Warm-up (15 minutes) a. What do you know about rocks? b. Everyone write down 5 things they know about rocks, then share. We will make a web on the board using what they know c. Minerals are the building blocks of rocks i. Can we always see the mineral crystals? NO…sometimes they are two small d. What are the 3 types of rock. How do they form i. Igneous: form when magma cools 1. Analogy: think about melted sugar. When I let it cool it forms crystals ii. Sedimentary: forms when sediments are eroded, travel, and are cemented together 1. Analogy: think about crystalized sugar. What happens if I let water get into the sugar? It forms little sugar clumps iii. Metamorphic: form under extreme heat and pressure. 1. Visualize: we have two plates pushing against each other and to form mountains. The rock is pushed together so much it forms metamorphic rock e. IF TIME show Rock Cycle Rap http://www.youtube.com/watch?v=rkGVE6wNAzo 2. Igneous Rocks (20 minutes) a. how are igneous rocks formed? i. Igneous rocks form when magma (molten rock) is forced up into the crust (lava is molten rock at the surface) Crystalization of magma b. where do igneous rocks form? i. On the Earth’s surface (extrusive rock= volcanic) 1. erupts out of rifts, and volcanoes ii. inside the Earth in little “pockets” (intrusive rocks) 1. magma gets stuck in the “magma chamber” under the earth’s surface and cools c. ACTIVITY: Put up Rock Comparison: How can we distinguish between rocks? i. Have each student spend 2 minutes comparing the rocks 1. Consider: colors, “grain size”, how many minerals they see, how heavy do you think they are ii. write queue words on the board: 1. fine-grained, course-grained, bubbles in it (why would there be bubbles? Escaping gas), rocks in a “fine-grained matrix” iii. USE Granite and Basalt iv. Write observations on the board… what are two characteristics we can use to distinguish them? 1. Grain size: it tells us how quickly/slowly the magma cooled. FAST=small grains, SLOW=large grains 2. Color: light vs. dark. This can tell us about the chemical composition d. ACTIVITY: Put up a slide texture, grain size, color. Put 2 more rocks on the board. (resource: http://geology.com/rocks/igneous-rocks.shtml ) i. Make observations: ESPECIALLY color and texture (glassy, fine-grained, large-grained, bubbly…_) ii. use RHYOLITE and OBSIDIAN iii. How grain size indicated by where formed? 1. Extrusive: fine grain, no time for crystals to grow 2. Intrusive: large grain size, crystals have time to grow 3. Cool down (5 minutes) a. Thumbs up/thumbs down game i. Igneous rocks are formed form crystallized magma. UP ii. Igneous rocks can only form on the earth’s surface. DOWN iii. Course-grained rocks are formed when magma cools really quickly DOWN iv. Dark colored rocks are more like continental crust DOWN v. Light colored rocks are more like continental crust UP vi. Minerals are the building blocks of rock UP JAPAN: GESCIENCE HOMEWORK SHEET DUE THURSDAY JUNE 28 Igneous Worksheet NAME _____________________________________________________________________________________ 1. List the three types of rocks, and write a sentence about how each is made. 2. DRAW, to the best of your ability, two rocks: (1) this rock cooled slowly, and is like continental crust in composition. (2) this rock cooled super-fast, and is like oceanic crust in composition. THURSDAY June 28- igneous rock continued and chemical composition MATERIALS Chalk and chalkboard Smart board and photos Typed on smart board END SLIP Scrap paper for end-slips OBJECTIVES Students will recognize trends igneous rocks due to their chemical composition, and use it to determine how rocks will act under stress. Students will summarize how magma moves up through the Earth’s crust, and how it changes as it moves to the Earth’s surface. Students will infer how intrusive FELSIC rocks are exposed on the surface using previous knowledge. PROCEDURE 1. warm-up a. AGENDA: today we are going to talk about Rock composition, and then what happens to magma as it moves through the Earth’s crust. b. Characterize the two rocks on the board 2. Composition… make a web a. What are the two types of lithosphere or crust? Continental and Oceanic b. What is the main type of rock on continental crust? Oceanic Crust? c. Why are they different? They are made of different minerals and have different chemical compositions d. To determine chemical composition we must use a scale e. the 4 areas are FELSIC/granatic, Intermediate/andesitic, MAFIC/basaltic, and ULTRAMAFIC i. FELSIC: feldspar and silica rich 1. Example minerals: quartz, k-feldspar ii. MAFIC: magnesium and iron rich 1. Example minerals: Olivine, pyrocene, amphibole iii. how do you think we can distinguish between the two? 1. COLOR and density 2. MAFIC darker, FELSIC lighter greys and pinks 3. The color has to do with the minerals each rock contains f. composition trends…put up on the blackboard. Fill in arrows as students guess answers: COMPOSITION TRENDS chart FELSIC INTERMEDAITE MAFIC ULTRAMAFIC --------------------------------- silica -------------------------------------------------------------------------Mg, Fe ---------------------------------- --------------------------------------density--------------------------------- ------------------------------viscosity-------------------------------------- -----------------------------melting temp --------------------------------- 3. how does magma change as it moves through the Earth’s crust, and how does it solidify? a. THINK PAIR AND SHARE b. Resource http://www.youtube.com/watch?v=SteA4FQxrO4&feature=related c. Magma moves up through the Earth’s continental crust d. As it moves it sometimes gets caught in a magma chamber e. Which type of mineral do you think would form crystals first? Consider melting temperature i. Minerals with a higher melting temperature crystalize first (ULTRA then MAFIC then INTER then FELSIC). f. Put up DIAGRAM of Bohen’s reaction and ask about it g. Lets say we have MAFIC magma moving up through continental crust. What do you think happens to the magma’s composition? Does it stay the same or does it change? i. Assimilation: when magma comes up through the crust, it melts surrounding rock changing the composition of the magma. For example MAFIC magma will melt continental crust it travels through (FELSIC), making the magma more silica rich and FELSIC ii. ANALOGY: what does it mean if an immigrant assimilates to the USA, or if a student from a middle school in California assimilates to a middle school in Pennsylvania? 1. They start to blend their old culture with the new one, becoming more of an intermediate between the two iii. ANALOGY: now lets take baking chocolate and heated heavy cream. What would happen? iv. Resource: http://www.youtube.com/watch?v=A5LdZJiKsVk&feature=rel ated starting at 5.20 4. THINK PAIR AND SHARE we know Granite, a FELSIC rock is intrusive. So how did it come to the Earth’s surface? How can we see it? What process occurred?...EROSION 5. wrap up: (5 minutes) a. End Slip activity: ask them to take out a paper and pencil. Put questions on the board b. c. d. e. How does igneous rock form? What are two characteristics we can look at to classify igneous rocks? What do MAFIC and FELSIC refer to? How does magma change as it moves through country rock? What is the process called? JAPAN: GESCIENCE HOMEWORK SHEET DUE FRIDAY JUNE 29 Igneous ID WORKSHEET NAME _____________________________________________________________________________________ Write the texture, grain size, color, and density of each rock in order to identify it. Use the chart below to help you. 1. Observations: Rock name____________________________________ 2. Observations: Rock name: _______________________________________ 3. Observations: Rock name: _______________________________________ 4. Observations: Rock name: ________________________________________ FRIDAY June 28- volcanoes MATERIALS Smartboard and YouTube Sticker Scrap paper OBJECTIVES Students will distinguish between FELSIC and MAFIC volcanic eruptions, considering chemical composition and characteristics. Students will order the steps of the Mount Saint Helen’s eruption. PROCEDURE 1. warm-up: a. http://www.youtube.com/watch?v=ZYEopZ4VhGs dramatic 1 minute video of lava flow to get kids “in the mood” to talk about volcanoes. Have playing as kids coming in and taking out homework b. tell me everything you know about volcanoes (pair and share) i. answers will be covered in the lesson ii. make sure they recap from yesterday that 1. volcanoes 2. Guess where a volcano occurs based on the type of explosion a. Remind students of the different properties of MAFIC and FELSIC rocks, and how MAFIC rocks are basaltic and FELSIC rocks are often granatic. Students should deduce that, basically, MAFIC volcanoes are in the ocean or volcanic islands, and FELSIC are in continents. FELSIC eruptions would be more explosive, and MAFIC are more fluid. b. Put a slide up on the board of a volcano and ask students to look at the picture of the type of volcano and flow produced, and what the composition of the lava is c. Answer by table: whichever wins gets a sticker 3. characteristics of intrusive and extrusive volcanoes, write on board and then go back to slides and try to re-identify When magmas reach the surface of the Earth they erupt from a vent called a volcano. They may erupt explosively or non-explosively. • Non-explosive eruptions are favored by low gas content and low viscosity magmas (basaltic to andesitic magmas and sometimes rhyolitic magma). ◦ Usually begin with fire fountains due to release of dissolved gases ◦ Produce lava flows on surface ◦ Produce Pillow lavas if erupted beneath water • Explosive eruptions are favored by high gas content and high viscosity (andesitic to rhyolitic magmas). ◦ Expansion of gas bubbles is resisted by high viscosity of magma – results in building of pressure ◦ High pressure in gas bubbles causes the bubbles to burst when reaching the low pressure at the Earth's surface. ◦ Bursting of bubbles fragments the magma into pyroclasts and tephra (ash). ◦ Cloud of gas and tephra rises above volcano to produce an eruption column that can rise up to 45 km into the atmosphere. 4. Continental Volcano example a. Mt. Saint Helens, Washington—1980 Video: http://www.youtube.com/watch?v=3XYfBxdVDJE describing eruption: use first 2 minutes, the events leading up to explosion Eruption: http://www.youtube.com/watch?v=fmsxmbVYMHo Leading events: plug Series events: 1. earthquakes 2. landslides (150 mi/hour) 3. lateral blast (300 mi/hour): 3 zones when hot are and gas pushed forward, trees singes and blown over in direction blast moving 4. pyroclastic flow (hot material and gas flowing down mountain) 5. mudflows/LAHARS 6. ash fall: ash pumped high into air…vertical eruption column o rock more felsic in continental volcanoes o more silica, higher viscosity…more likely to explode (because can’t flow) o Volcanic dome formed…plugging up vent o Plug…when gas explodes get ash fall (pyroclastic flow) Pyroclasts: ash, particle emitted from a volcano Pyroclastic flow: hot material and gas flowing down the mountain o Volcanic ejecta: volcanic bomb (> 64 mm), ash flow (weld together to ground to get welded tuff), volcanic breccia ( ~ .03 mm) cinder (2 mm-64mm) 5. Hot-spots a. Can happen in the ocean (like the Hawaiian islands) or in continental crust (Yellowstone hotspot) b. Hot spots: where hot material rises up (in mantle plume) and forms a volcano. flow from decompression melting 6. cool-down a. What is the cooled thing about volcanoes you have learned today? Go around class and answer