Answer Key - Changes in Matter
advertisement
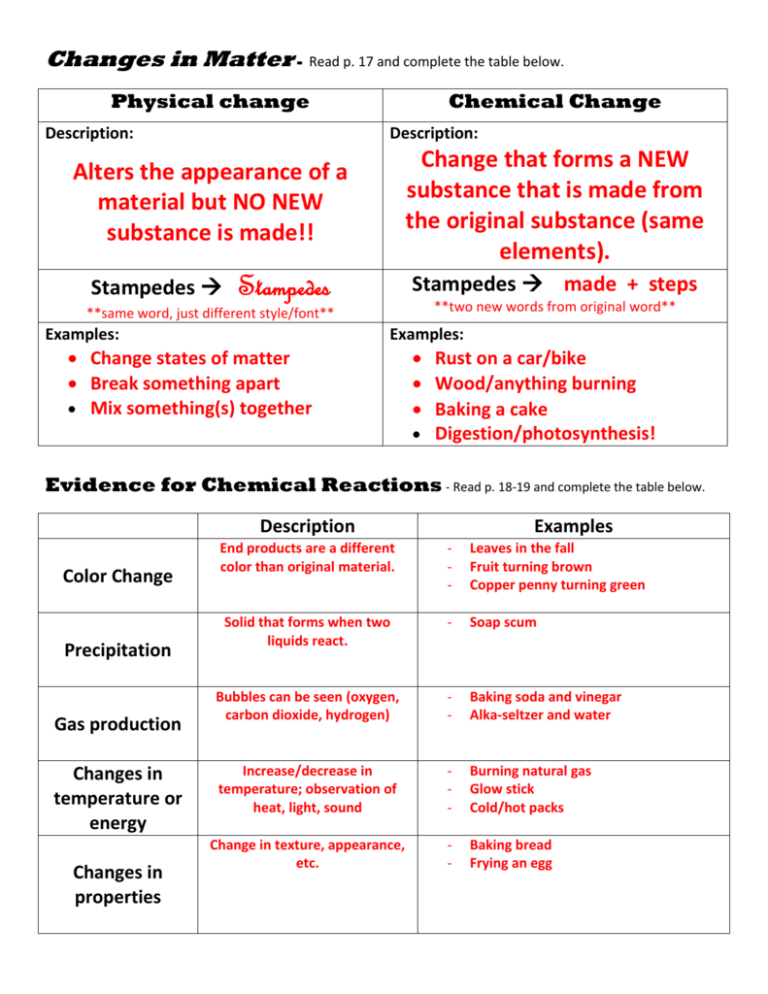
Changes in Matter - Read p. 17 and complete the table below. Physical change Description: Chemical Change Description: Change that forms a NEW substance that is made from the original substance (same elements). Alters the appearance of a material but NO NEW substance is made!! Stampedes Stampedes Stampedes made + steps **two new words from original word** **same word, just different style/font** Examples: Examples: Change states of matter Break something apart Mix something(s) together Rust on a car/bike Wood/anything burning Baking a cake Digestion/photosynthesis! Evidence for Chemical Reactions - Read p. 18-19 and complete the table below. Description Color Change Precipitation Gas production Changes in temperature or energy Changes in properties Examples End products are a different color than original material. - Leaves in the fall Fruit turning brown Copper penny turning green Solid that forms when two liquids react. - Soap scum Bubbles can be seen (oxygen, carbon dioxide, hydrogen) - Baking soda and vinegar Alka-seltzer and water Increase/decrease in temperature; observation of heat, light, sound - Burning natural gas Glow stick Cold/hot packs Change in texture, appearance, etc. - Baking bread Frying an egg Observing Chemical Change – Review and Reinforce Part A: Classify the following changes as either physical or chemical changes. Explain each answer. 1. A piece of paper is cut into many pieces. PHYSICAL – still paper, just in smaller pieces. 2. A piece of paper is set on fire. CHEMICAL – color change, energy release. 3. A sidewalk is warmed by the heat of the sun. PHYSICAL – still sidewalk, just warmer! 4. Salt crystals appear when a glass of saltwater is left on a windowsill for several days. PHYSICAL – water evaporates (state change) leaving salt behind. 5. A can of soda tastes flat after being left open for several hours. PHYSICAL – all the dissolved CO2 escapes liquid. 6. A piece of ice melts into a puddle of water. PHYSICAL – state change (solid to liquid). 7. A copper bracelet turns green. CHEMICAL – color change with new material produced. 8. A piece of banana turns brown after being cut. CHEMICAL – color change on outside of fruit. 9. A plant uses sunlight to produce stored energy. CHEMICAL – new material made (sugar is stored energy). Part B: Complete the following table. Describe changes in properties that you might notice during each process and state whether the changes are chemical or physical. Changes in Matter Event Baking a cake Burning a log Freezing water Observable Changes Type of Change 10. “liquid” sticky mixture changes into spongy, fluffy cake. 11. chemical 12. solid wood (light in color) change to ash (dark to white in color); energy (heat/light) released. 13. chemical 14. liquid water solidifies into ice (phase change). 15. physical Chemical Interactions Part A: Valence electrons and Electron dot diagrams - Use the examples in Figure 6 on p. 57 to draw electron dot diagrams for the following elements. Element Symbol # valence electrons E-dot diagram Element Chlorine Cl 7 Cl Phosphorus P 5 Oxygen O 6 Argon Ar 8 Sodium Na 1 Aluminum Al 3 Barium Ba 2 Silicon Si 4 Part B: Symbol # valence electrons E-dot diagram How many of each element is needed?? – Use the examples above to help you determine how many of each element would be needed to make ‘happy’ atoms! Remember, elements in the same group (column) have the same number of valence electrons (i.e. their E-dot diagrams would look the same!!) Elements Number of each Formula (p.25) Sodium + Chlorine 1 1 NaCl Barium + Oxygen 1 1 BaO Aluminum + Fluorine 1 3 AlF3 Magnesium +Phosphorus 3 2 Mg3P2 Potassium + Sulfur 2 1 K2S Aluminum + Oxygen 2 3 Al2O3 Lithium+ Bromine 1 1 LiBr Hydrogen + Oxygen 2 1 H2O Beryllium + Iodine 1 2 BeI2 Aluminum + Nitrogen 1 1 AlN Structure of a Chemical Equation Reactants Reactants (p. 26) Products Products Conservation of Mass and Balanced Chemical Equations (p. 26-28) Part A: Writing Chemical Formulas - Use your knowledge from the previous section to write the chemical formula for each element combination. F H N S Br O HF H3N H2S HBr H2O Na NaF Na3N Na2S NaBr Na2O Mg MgF2 Mg3N2 MgS MgBr2 MgO Ca CaF2 Ca3N2 CaS CaBr2 CaO Al AlF3 AlN Al2S3 AlBr3 Al2O3 Part B: Balancing Chemical Equations – Use some of the examples from above to write and balance chemical equations. **Did not complete in class** Reactant + Reactant Yields H + O Al + S Na + Br Mg + F Ca + S Na + O H + Br Na + N Al + F Product (formula) Identifying Balanced and Unbalanced Equations Part A: Identify which equations below are balanced (B) and which are unbalanced (U) by writing the corresponding letter on the line. Part B: If the equation is UNBALANCED, then use the space provided to balance the equation. _________1. Pb(NO3)2 + 2KI PbI2 _________2. 2H2 + O2 2H2O Balanced 3. CuSO4 + Zn ZnSO4 _________4. ZnS + 2O2 ZnSO4 Balanced 5. 2NaOH + CO2 Na2CO3 Balanced 6. H2 + Cl2 2HCl _________7. 2Mg + O2 2MgO _________8. 2Na + 3H2O Balanced 9. NaOH + HCl _________10. N2 + _________11. 2Al _________12. _________13. + 2KNO3 + Cu + H2 O 2H2 + 2NaOH NaCl + H2 O 3H2 2NH3 + 3H2SO4 Al2(SO4)3 + 3H2 As2O5 + 3H2O 2H3AsO4 2C + O2 2CO Classifying Chemical Reactions Type of Reaction Synthesis (p. 29-31) Description General example Specific example(s) When two or more substances combine to make a more complex substance. A + B AB 2Na + Cl2 2NaCl CaO + H2O Ca(OH)2 ONE PRODUCT!! Decomposition Breakdown of compounds into simpler products. AB A + B 2H2O2 2H2O + O2 2NaN3 2Na + 3N2 ONE REACTANT!! When one element replaces another in a compound. Replacement (single) 2 REACTANTS 2 PRODUCTS Replacement (double) 2 REACTANTS 2 PRODUCTS **Always have one single/lone element on reactant/product side** When two elements in different compounds change place. Fe + CuSO4 Cu + FeSO4 A + BX B + AX 2Na + 2H2O 2NaOH + H2 AB + XY XB + AY HCl + NaOH NaCl + H2O MgCl2 + K2S MgS + 2KCl **Always have compounds on reactant/product sides** Balancing Synthesis Reactions – balance the following examples of synthesis reactions. 1. P4 + 5O2 P4O10 2. 2H2 + O2 2H2O 3. NaOH + CO2 Na2CO3 4. 2CO + O2 2CO2 5. K2S + 2O2 K2SO4 6. 2Li + Cl2 2LiCl + H2 O Balancing Decomposition Reactions – balance the following examples of decomposition reactions. 1. 2HClO O2 + 2HCl 2. CaC2O4 Ca + 2CO2 3. PtCl4 Pt + 2Cl2 4. 2H2O2 2H2O + O2 5. 2BaO2 2BaO + O2 6. 2KClO3 2KCl + 3O2 Identifying and Balancing Replacement Reactions Part A: Identify the following equations as either single replacement (SR) or double replacement (DR). Part B: Balance each of the equations of replacement reactions below. *Balanced SINGLE 1.* SnCl2 + Fe FeCl2 + Sn DOUBLE 2.* CoCl2 + Ca(OH)2 CaCl2 + Co(OH)2 SINGLE 3. 2HCl + Cu CuCl2 + H2 DOUBLE 4. CdCl2 + H2 S CdS + 2HCl DOUBLE 5. BaCl2 + K2CrO4 BaCrO4 + 2KCl DOUBLE 6. PCl3 + 3AgF PF3 + 3AgCl SINGLE 7.* CuSO4 + Zn ZnSO4 + Cu SINGLE 8. 2HNO3 + Ba Ba(NO3)2 + H2 SINGLE 9. 3MnO2 + 4Al 2Al2O3 3Mn + Identifying and Balancing Chemical Reactions Part A: Identify each of the following reactions as Synthesis (S), Decomposition (D), Single Replacement (SR), or Double Replacement (DR). Part B: Balance each of the equations below if needed. (*6 are balanced!!) Double R 1. CoCl2 + H2 O CoO + 2HCl Single R NiO2 + C CO2 + Ni 2.* Double R 3. Pb(NO3)2 + 2NaCl PbCl2 + 2NaNO3 Double R 4.* HCl + NaOH NaCl + H2 O Decomp 5.* CaCO3 CaO + CO2 Single R 6. CoF3 + 3S 3SF + Co Synthesis 7.* Mg + Br2 MgBr2 Double R 8. NiSO4 + 2NaOH Ni(OH)2 + Na2SO4 Synthesis 9.* MoO3 + MnO MnMoO4 Single R 10. C2H2O4 + 2Na Na2C2O4 + H2 Decomp 11.* Cu(OH)2 CuO + H2 O Double R 12. 2HF + Li2CO3 2LiF + H2CO3 Double R 13. CaCl2 + 2AgNO3 Ca(NO3)2 + 2AgCl Controlling Chemical Reactions - Read p.32-34 and complete the table. Exothermic Reaction Endothermic Reaction Description: A reaction that releases energy in the form of heat. Description: A reaction which absorbs energy (absorbs heat…feels cold!) EXO OUT!! (to release) ENDO IN!! (take in) Examples: Examples: Gasoline + oxygen Baking soda + vinegar Activation energy – the minimum about of energy needed to start a chemical reaction. Rates of Chemical Reactions – Read p. 34-37 and complete the table below. The rate (how fast or slow) of a chemical reaction is affected by such factors as concentration, surface area, and temperature, and by using substances called catalysts and inhibitors. Factor Description Concentration The amount of one material in a given Effect on reaction Surface area The amount of exposed surface of a material. Increase concentration, increase particles available to react. Increase surface area, increases rate of reaction. Temperature Change temperature changes how fast particles move (increase contact and increase energy) Increase temp, increase reaction rate; decrease temp., decrease reaction rate. volume of another material. Catalysts Material that increases the rate of reaction by decreasing the activation energy (NOT permanently changed…not a reactant!) Add catalyst, increase reaction rate. Inhibitors Material used to decrease the rate of a reaction. Add inhibitor, decrease reaction rate. Packet p. 21 – Answer Key 1. batter rises (gas produced) and sticky batter turns fluffy. 2. chemical 3. wood turns to ash (black/white in color); heat/light energy produced. 6. physical 7. physical change 8. matter 9. exothermic 10. endothermic 4. chemical 11. chemical reaction 5. state change from liquid to solid 12. precipitate 13. chemistry Packet p. 22 – Answer Key 1. FeS + HCl FeCl2 + H2S 6. c 2. Na + F2 NaF 7. g 3. HgO Hg + O2 8. f 4. 2 molecules of Hydrogen (H2) and 1 molecule of Oxygen (O2) produces 2 molecules of water (H2O) 9. a 5. the reactants include 4 atoms of Hydrogen (2H2) and 2 atoms of Oxygen (O2) and the products are the same – 4 atoms of Hydrogen with 2 atoms of Oxygen (2H2O). 12. b 10. e 11. h 13. d






