Preventing the clinical manifestations (PPTX 2.7MB)
advertisement

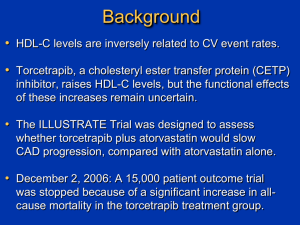
Preventing the Clinical Manifestations of CV Disease in Asia: Opportunities for Lipid Management Slides prepared and presented by Prof John JP Kastelein Academic Medical Centre Amsterdam, The Netherlands The Burden of CVD in Asia: CHD Deaths by Country, 2002 WHO CVD Atlas. 2002. WHO Stroke Atlas. 2002. 2 The Burden of CVD in Asia: Stroke Deaths by Country, 2002 WHO CVD Atlas. 2002. WHO Stroke Atlas. 2002. 3 Age-Standardized Stroke and CHD Death Rates by Country, 2002 Ueshima H et al. Circulation. 2008;118:2702-2709. 4 Projected Stroke and CHD Increase to 2030 in China Moran et al. Circ Cardiovasc Qual Outcomes. 2010;3;243-252. 5 Increase in Age-Standardized Mean Total Cholesterol Levels in Asia 1980-2008 Men Women Southeast Asia: Cambodia, Indonesia, Lao People’s Democratic Republic, Malaysia, Maldives, Myanmar, Philippines, Sri Lanka, Thailand, Timor-Leste, Vietnam East Asia: China, Hong Kong (China), Macau (China), Democratic People’s Republic of Korea, Taiwan, Brunei, Darussalam, Japan, Republic of Korea, Singapore, islands of Oceania Farzadfar et al. Lancet. 2011;377:578-586. 6 Discovery of statins Discovery of LDL receptors Brown and Goldstein, 1974 Endo, 1976 Statins raise LDL receptors in the liver Plasma LDL is reduced Clear Cardiovascular Benefits of Intensive Lipid-Lowering Therapy POSCH-PL % Patients with CHD Event 4S-PL Primary prevention trials Secondary prevention trials HPS 25 POSCH-Rx CARE-PL 20 4S-Rx 15 10 LIPID-PL TNT-10A CARE-Rx WOSCOPS-PL LIPID-Rx TNT-80A WOSCOPS-Rx HPS-Rx LRC-PL 5 ASCOT-PL ASCOT-Rx 0 Statin trials HPS-PL non statin trials LRC-Rx AFCAPS-PL AFCAPS-Rx 50 70 90 110 1.3 1.8 2.3 5.4 (mmol/L) 130 2.8 150 170 190 210 (mg/dL) 3.4 3.9 4.4 4.9 LDL cholesterol Second CTT cycle: More vs less intensive statin therapy Study Treatment comparison N Target population Entry lipid criteria PROVE-IT A 80 vs. P 40 4162 ACS TC ≤240 mg/dL A to Z S 40 then S 80 vs. placebo then S 20 4497 ACS TC ≤250 mg/dL TNT A 80 vs. A 10 10,001 Prior CHD LDL-C 130-250 mg/dL TG ≤600 mg/dL IDEAL A 80 vs. S 20-40 8888 Prior CHD TG ≤600 mg/dL SEARCH S 80 vs. S 20 12,064 Prior CHD TC ≥4.5 mmol/L or ≥3.5 if on statins Proportional effects on MAJOR VASCULAR EVENTS of events (% pa) per mmol/L reduction inNo. LDL cholesterol Statin/ Control/ More statin Less statin Relative risk (CI) 3485 (1.0) 1887 (0.5) 5105 (1.4) 4593 (1.3) 2281 (0.6) 6512 (1.9) 0.73 (0.69 - 0.78) 0.80 (0.74 - 0.87) 0.76 (0.73 - 0.78) 1453 (0.4) CABG 1767 (0.5) PTCA Unspecified 2133 (0.6) Any coronary revascularisation 5353 (1.5) 1427 (0.4) Ischaemic stroke Haemorrhagic stroke 257 (0.1) 618 (0.2) Unknown stroke Any stroke 2302 (0.6) 1857 (0.5) 2283 (0.7) 2667 (0.8) 6807 (2.0) 1751 (0.5) 220 (0.1) 709 (0.2) 2680 (0.8) 0.75 (0.69 - 0.82) 0.72 (0.65 - 0.80) 0.76 (0.70 - 0.82) 0.75 (0.72 - 0.78) 0.79 (0.72 - 0.87) 1.12 (0.88 - 1.43) 0.88 (0.76 - 1.01) 0.84 (0.79 - 0.89) Any major vascular event 10973 (3.2) 13350 (4.0) 0.78 (0.76 - 0.80) Nonfatal MI CHD death Any major coronary event 99% or 95% CI 0.4 0.6 0.8 Statin/more statin better 1 1.2 1.4 Control/less statin better Absolute effect of statin therapy on MAJOR VASCULAR EVENTS 15 Statin 15% relative risk reduction per 0.5 mmol/L 10 More statin 21% relative risk reduction per mmol/L 5 Combined evidence: ~33% relative risk reduction per 1.5 mmol/L 0 Five year risk of a major vascular event, % 20 Control 0 1 2 3 LDL cholesterol, mmol/L 4 5 ASAP: Atorvastatin Reduced CRP to a Greater Extent Than Simvastatin Baseline 0 Change (%) -10 1 year 2 years 14.0 19.7 -20 P<.001 -30 P<.022 -40 40.1 -50 44.9 Atorvastatin 80 mg Simvastatin 40 mg van Wissen S et al. Atherosclerosis. 2002;165:361-366. Additional Findings • No correlation between CRP and LDL-C reduction • Significant correlation between decrease in CRP and reduction in IMT (r =.13; P=.03) • Patients in the highest tertile of change in CRP had the greatest mean reduction in IMT Anglo-Scandinavian Cardiac Outcomes Trial— Lipid Lowering Arm (ASCOT-LLA): Study Design • • • • • Patient population Men and women aged 40-79 years Untreated HTN (SBP 160 mm Hg, DBP 100 mm Hg, or both) 19,342 10,305 patients patients with with HTN TC 251.4 mg/dL Treated HTN (SBP 140 mm Hg, DBP 90 mm Hg, or both) TC 251.4 mg/dL Atorvastatin 10 mg (n=5168) Placebo (n=5137) 5 years •Trial stopped at 3.3 years, 2 years earlier than expected At least 3 additional Primary CVD risk factors efficacy end point • Nonfatal MI, including silent MI, and fatal CHD HTN=hypertension; SBP=systolic blood pressure; DBP=diastolic blood pressure; TC=total cholesterol; CVD=cardiovascular disease. Sever PS et al. Lancet. 2003;361:1149-1158. ASCOT-LLA: Atorvastatin Reduced the Occurrence of First Major CV Events Patients with nonfatal MI and fatal CHD (%) 4 36% RRR in nonfatal MI and fatal CHD P=.0005 3 Placebo 2 Atorvastatin (10 mg) 1 0 0.0 0.5 1.0 1.5 2.0 Years RRR=relative risk reduction. Adapted from Sever PS et al. Lancet. 2003;361:1149-1158. 2.5 3.0 3.5 PROVE IT: Study Design Patient population • Men and women aged 18 years • • • Hospitalized within 10 days of acute MI or highrisk unstable angina (UA) TC 240 mg/dL Atorvastatin 80 mg (n=2099) 4162 patients Stable condition, enrolled after percutaneous coronary intervention (PCI), if planned Pravastatin 40 mg (n=2063) 18 to 36 months Primary efficacy end point • Composite of death from any cause, MI, documented UA requiring rehospitalization, revascularization, and stroke Cannon CP et al. N Engl J Med. 2004;350:1495-1504. Death, MI, urgent revascularization (%) PROVE IT: Significant Clinical Benefit With Atorvastatin Occurred as Early as 30 Days Composite end point of death, MI, or urgent revascularization 4 Pravastatin (40 mg) 3 33% RRR P=.043 2 Atorvastatin (80 mg) 1 0 0 5 10 15 20 25 Time after entry to trial (days) Adapted from Cannon CP et al. Circulation. 2004;110(suppl III);III-499. 30 Atorvastatin Is the Only Statin With an Active HMG CoA Reductase Inhibitor Metabolite Atorvastatin parent molecule CH3 CH3 CH O .. NHC N OH OH O O F Active ortho-hydroxy-atorvastatin metabolite 70% of the activity of atorvastatin is attributed to active metabolites Site* H O O N O H3C H CH3 OH OH .. N H *Unique to ortho-hydroxy metabolite. Data on file (RP Mason). Pfizer Inc., New York, NY. F O OH What Accounts for the Added Benefits of Atorvastatin? • Endothelial effects • Anti-inflammatory effects Reduction of lipids + • Antioxidant effects • Reduction in plaque progression • Plaque stabilization Wassmann S, Nickenig G. Endothelium. 2003;10:23-33. Statin Safety in Perspective Number needed to treat for 1 year to: Cause a GI Bleed1 Aspirin Cause a Fatal GI Bleed1 248 Cause Severe Myositis2 Statins 100,000 2066 Cause Fatal Myositis2 1,000,000 1Derry S, Loke YK. 2000 2Thompson PD, et al. 2003 Safety of Atorvastatin 80 mg in Clinical Trials Follow-up Patients ALT/AST >3x ULN* CK >10x ULN* Newman et al† variable 4798 26 (0.6%) 2 (0.06%) PROVE-IT 2 years 2099 69 (3.3%) NA TNT 4.9 years 4995 60 (1.2%) 0 IDEAL 4.8 years 4439 61 (1.38%) 0 SPARCL 4.9 years 2365 51 (2.2%) 2 (0.08%) variable 18,696 267 (1.43%) 4 (0.021%) Total *Consecutive measurements. †Newman C et al. Am J Cardiol. 2006;97:61-67; Cannon CP et al. N Engl J Med. 2004;350:1495-1504; LaRosa JC, et al. N Engl J Med. 2005;352:1425-1435; Pedersen TR et al; for the IDEAL Study Group. JAMA. 2005;294:2437-2445; Amarenco P et al. N Engl J Med. 2006;355:549-559. 20 Is Current LDL Reduction Enough? 700 600 CV events 500 placebo treated 30.6% reduction 400 31.0% reduction 300 200 100 0 4S 2º prevention trial with simvastatin WOSCOPS 1º prevention trial with pravastatin The Future of Best Practice “Normal” plasma cholesterol 700 (18.0) 300 (7.7) - - Physiologic level for plasma LDL-cholesterol as predicted from receptor studies 25 mg/dL (0.65 mmol/L) FH homozygotes FH heterozygotes 200 (5.2) - 150 (3.9) - 100 (2.6) - 50 (1.3) - 0 Guinea pig Sheep Rat Rabbit Cow Camel Pig Normal adults Newborns REVERSAL: Benefit of Intensive LDL-C Lowering on Plaque Progression 3 Progression (P=0.001) pravastatin 40 mg Percent change in atheroma volume 2 1 atorvastatin 80 mg P=0.02 between treatment groups 0 No change (P=0.98) -1 Nissen SE et al. JAMA 2004;291:1071–1080 REVERSAL Comparison of % LDL Cholesterol Reduction and Change in Atheroma Volume 20 Change in Atheroma Volume, mm3 15 10 5 0 50% LDL-C reduction -5 -10 -15 -80 -70 -60 -50 -40 % Change in LDL Cholesterol -30 -20 -10 0 10 20 REVERSAL: Intensive Lipid Lowering With Atorvastatin Halted Plaque Progression After 18 Months Change in atheroma volume (mm3) 20 15 10 Pravastatin (40 mg) 5 0 Atorvastatin (80 mg) -5 -10 -15 -80 For any degree reduction in LDL-C, the progression rate was lower with atorvastatin than with pravastatin -70 -60 -50 -40 -30 -20 -10 Change in LDL-C (%) Nissen SE et al. JAMA. 2004;291:1071-1080. 0 10 20 Age-Standardized Event Rate (per 100 Person-Yr) Relationship Between Estimated GFR (eGFR) and Clinical Outcomes Death from Any Cause Total Events = 51,424 Cardiovascular Events Total Events = 139,011 Any Hospitalization Total Events = 554,651 16 40 160 14 35 140 12 30 120 10 25 100 8 20 80 6 15 60 4 10 40 2 5 20 0 0 0 ≥60 45–59 30–44 15–29 <15 ≥60 45–59 30–44 15–29 <15 ≥60 45–59 30–44 15–29 <15 eGFR (mL/min/1.73 m2) Go AS, et al. N Engl J Med. 2004;351:1296-305. 26 % of patients with change in eGFR Proportion of Patients With Decline or Improvement From Baseline eGFR 50 Atorvastatin 10 mg P<0.0001 Atorvastatin 80 mg 45.6% 40 37.8% 30 20 10 0 P<0.0001 9.2% 6.6% (n=3324) (n=3225) (n=1505) (n=1602) eGFR decline from ≥60 mL/min/1.73 m2 eGFR improvement from <60 mL/min/1.73 m2