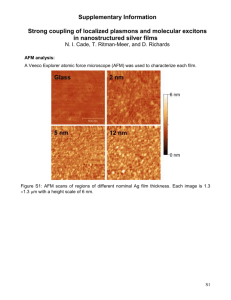
UIUC Demo Reminder
Ian Armstrong
March 12, 2016
Samples and Prep
• Celgard (Bruker sample)
• DNA
• “NanoDiscs”
• MoS2
• Customer Calibration Grid
Celgard sample was run a few times as a “standard” sample.
Most of the customer samples were various forms or DNA. As experienced
during the demo, the most crucial part of taking good images of these is the
sample preparation. Included in the next slide is sample preparation we use
ourselves, but note there are a fair few different protocols to be found in the
literature, and if the sample is substantially different from that studied before it
might necessarily require a different procedure.
“NanoDiscs” previously studied via an old MultiMode AFM were imaged.
Noise tests were conducted on an MoS2 sample.
Speed tests were conducted on a calibration grid.
DNA Sample Prep
Attached is the procedure we use to prepare DNA samples on mica in fluid. The recipe for the
incubation/adsorption buffer is on the first page (attached .pdf). For adsorbing the chromatin you can jump
to the second page to the section ‘On day of Experiment…’ (we have the steps in between as our DNA
comes as a very concentrated solution that we dilute and store in the EDTA-containing buffer. The final
dilution into the HEPES/NiCl2 buffer gives us a final concentration of 0.5 microgram/mL (others have used
1 microgram). You can replace the NiCl2 in this buffer with MgCl2.
Briefly,
You want to transfer/dilute your chromatin into your MgCl2 or NiCl2 containing buffer.
Pipette an aliquot of the Chromatin in MgCl2/NiCl2 containing buffer onto a freshly cleaved mica surface
(we use ~100 micro-L, depending on the size of your mica disk – you can use 50 micro-L if you need less).
Allow it to adsorb for 10-15 minutes
At this point you can either image the surface directly – this will help you to see if your concentration is too
high/low or if you need to incubate longer. However, chromatin will continue to adsorb to the mica over
time – which can be messy. Alternatively, before you image you can rinse the sample with chromatin-free
HEPES/NiCl2 or MgCl2 buffer (For rinsing I usually pipette most of the droplet off the mica and then pipette
on fresh buffer – do this ~3 times) and then image in this buffer.
If you want to image in air, you will need to rinse away the buffer with ultra-pure or milli-Q water
(otherwise, once the buffer dries you’ll have beautiful salt xtals on your sample) and then gently remove
the water from the mica surface (I usually tip off most of the droplet by tilting the sample over the sink.
Then I’ll wick the remainder off the droplet slowly with the edge of a chem-wipe and allow the sample to
fully dry on a benchtop. The AP-mica is a little better for keeping the chromatin attached on the surface for
a dried sample. I find that sometimes, with the NiCl2 buffer when we dry the sample, removing the NiCl2
disrupts the interaction between the DNA and the mica and the DNA clumps up. This is why I try to dry as
slowly as possible.
I hope this sample preparation helps you. Please, feel free to call me if you have any questions. Good Luck!
Andrea Slade, Ph.D.
Life Science Applications Scientist andrea.slade@bruker-nano.com
Dimension Icon AFM
The Dimension Icon AFM is the latest generation of the worlds
most popular tip scanning AFM. Keeping the successful design of
previous generations which includes:
• flexibility of sample size
• easy customization
• motorised and automated controls for ease of use
• inherent stability and robust design
• huge accessory array for multiuser experiment flexibility
On top of this the Dimension Icon has a new
powerful controller (some details on next slide).
The isolation platform and hood has been
improved for even better stability and drift. The
AFM has multiple upgrades including, heavier
base, single Invar arm for increased space
underneath tip, digital camera optics, LCD
display, all new fully closed loop scanner with
closed loop performance almost
indistinguishable from open loop. The software
has also been dramatically improved for this
latest generation instrument. Neat experiment
selectors with preloaded parameters and cues
to help select appropriate probes. A probes
database to load nominal values of probe
parameters. Work flow models to aid novice
and infrequent users. Context sensitive help
menus, just hit F1 in realtime or offline
software for comprehensive help.
Dimension Icon AFM
AND: Peak Force Tapping, our latest
scanning technology only available
through Bruker. This technique makes
scanning easier and more intuitive as you
have the setpoint directly as a force in
Newtons. No “tuning” as in tapping mode.
We can scan at ultra low forces, even lockin on the snap-in or negative force. We
can vary force curve approach and
modulation to suit sticky/ rough samples
or for probe functionalisation experiments.
Through this technique we can use
ScanAsyst to automate gain/ scan rate/
setpoint for automated image
optimisation. Now we are also fully
leveraging this technique to look at
mechanical properties by evaluating the
force curves on the fly, or combining with
existing techniques such as conductive
AFM to be able to look at fragile or sticky
conductive samples not possible to be
imaged with standard CAFM. And more
things to come…
Nanocscope V Controller
• 8 Imaging Channels:
• 3 Built in Lock-In Amplifiers:
• 500kHz/ 50MHz sampling rate:
• Thermal Tune:
• High Speed data Capture:
• Digital Q Control:
• 5k x 5k pixel images:
• Digital Inputs/ Outputs:
• Signal Access Front Panel:
8 realtime channels capturing at up to 5Kx5K pixels each
2 high speed (1 kHz to 5 MHz) 1 low speed (0.1 Hz to 50 kHz)
Generic Lock-In GUI: easily route reference signal and lock-in input
Monitor Cantilever Harmonics for: enhanced phase, mech properties,
dC/dZ from surface potential
2us feedback response time = better tracking and tip wear
Identify resonant frequencies up to 2MHz, calculate spring constants
Capture 64Mb of data.
2 Channels At 50 MHz (AC coupled) ~330 ms per channel
2 Channels At 6.3 MHz
2 Channels At 500 kHz (DC Coupled) ~2.5 seconds per channel
Works In Image, Ramp And Sweep Modes, Not Required To Be Engaged
Trigger on EOL, EOF, Edge or Manual
Data collection can start before or after trigger event
Enhancements to 10x, damping to 10x, Q seek
Benefits Include:
improved signal-to-noise ratio in phase-contrast imaging MFM, EFM etc
faster scanning in fluid with damped Q
stay in attractive mode
better force control for soft samples
Scan large areas at hi pixels for better statistics but same resolution
2 Digital Outputs, end of line, end of frame
2 Digital Inputs, 3.3 Volts (5 Volt compatible) up to 25 MHz
e.g. Digital Photomultiplier, Photon-Counting CCDs, sync pulse
Access to lock-in amps, XYZ low voltage drive signals, input external data
User Access To Data Signals, height, amplitude, phase etc…
Dimension Icon Imaging Modes
Standard
1. ScanAsyst/ PFT
2. TappingModeTM (air)
3. Contact Mode
4. Lateral Force Microscopy
5. PhaseImagingTM
6. Lift Mode
7. MFM
8. Force Spectroscopy
9. Force Volume
10.EFM
11.Surface Potential
12.Piezoresponse Microscopy
Optional
1. PeakForce-QNM
2. HarmoniX
3. Nanoindentation
4. Nanomanipulation
5. Nanolithograpy
6. Force Modulation (air and fluid)
7. TappingMode (fluid)
8. Torsional Resonance Mode
9. Dark Lift
10. STM
11. SCM
12. C-AFM
13. SSRM
14. TUNA
15. VITA
Application Modules and Accessories
•
Dimension App Mods (electrical modes)
– DM-SSRM
– DM-CAFM
– DM-SCM
– DM-TUNAV-50/-60
•
Heating/Cooling
– Sample Heating and Cooling: -35C to 250C
– Sub-100 nm node heating (VITA)
•
Software
– Nanoacope V8 Realtime
– Nanoscope Analysis Offline
– Intuitive, comprehensive, context sensitive help
•
Cantilever Holders and Probes to support all modes,
manufactured by us
Working in Liquid: Hardware
To work in a liquid environment the only accessory needed
is the fluid cantilever holder (pictured left). This single
holder allows working in fluid in contact mode, tapping
mode, and Peak Force Mode and more…
Loading a probe to the holder is identical to air i.e. place
cantilever with tweezers under a spring loaded clip.
The fluid cantilever holder is then inverted and
attached to the scan head just as for the air
cantilever holder. The laser is aligned on the
cantilever as normal. The final step is to place the
sample on the sample holder and deposit ~50uL
via pipette to the sample. This forms a bubble on
the surface which the cantilever/ fluid holder is
then lowered into. The bubble then forms a liquid
tension seal around the circular portion of the
cantilever holder. The user is then ready to start
imaging. Pictured right is how it looks when
actually scanning in liquid.
Working in Liquid: Software
As well as optimized and simple hardware we
have dedicated fluid workspaces. This screen
dump shows one example of that. The fluid
workspaces have optimized engage routines/
scan parameters for liquid – also a very handy
refractive index autocompensate checkbox (to
account for different optical path when liquid is
introduced – this makes it easier to find the
focus position/ engage point). With our patented
Peak Force Tapping mode there is also no
need to tune the cantilever – a process
inherently less trivial in liquid than air.
Sample Images: Celgard
Celgard is the tradename for a form of
isotactic polypropylene that has been
stretched such that it has drawn fibrillar
sections at 90 degrees to crystalline
lamella. It is used as a separator or
filtration membrane. It is used as a
somewhat standard AFM sample
because you need:
1. Good force control and resolution to
clearly see the lamellae.
2. Good force control and feedback to
track the ~100nm deep freely
suspended fibrils.
3. And to be able to do this at
reasonable scan rates.
We routinely measure this sample in
tapping and Peak Force Tapping. Peak
Force Tapping is actually more beneficial
for this kind of sample. The image right
was taken on site directly after installing
the instrument.
Sample Images: DNA
Shown right are 4 images from various
groups that attended on the first day.
The image top left shows a more
standard DNA image. Here we pretty
much had the prep technique good.
There were a few large agglomerations
that interfered with the imaging but we
were nearly there. In spite of this you
can still see the crisp DNA imaging. The
other 3 DNA samples shown
incorporated various differences in the
sample such as chromatin. The affect
we observed seemed to be to kink the
DNA molecule and also at higher
concentrations it seemed to
preferentially form a network. Again,
once sample prep had been optimised
were able to scan at ~1um and obtain
good images.
500nm
500nm
500nm
500nm
Sample Images: NanoDiscs
Mark McLean’s samples of Nanodiscs. Mark
has been imaging these on an old Bruker
MultiMode AFM. We got to the same or
better results, quicker and he remarked
with less drift affecting the measurements.
The “discs” can be seen individually in
these images. They are just 10nm wide.
When they vary in height it can be
indicative of a bound protein. Image taken
with Peak Force Tapping at 30pN imaging
force in liquid.
http://sligarlab.life.uiuc.edu/nanodisc.html
200nm
200nm
Sample Images: MoS2 TAP Roughness
Icon Scanner
Here we are tracking
multiple steps on the
MoS2 sample. For
roughness evaluation we
first order flattened and
plane fit to the region as
indicated by the white
dashed box. In the image
it may be slightly unclear
due to color contrast but I
have highlighted in blue
dash 3 single atomic steps
~6A in height (see
original data for clear
view). We analyzed the
roughness in the white
box area. Ra value
~16pm
Sample Images: MoS2 PFT Roughness
Icon Scanner
Same thing again, this
time with Peak Force
Tapping mode. In this
instance we got 23pm Ra.
It should be noted that in
comparing one mode to
the other and as a basis
for instrument noise
whilst imaging – a more
statistical approach may
help doing multiple areas
on the sample. As we are
right at the business end
of the spectrum, very
small insignificant things
will affect the
measurement. We do not
believe PFT mode would
actually affect the noise of
the system.
Sample Images: MoS2 PFT Step Height
FastScanner
We repeated the process with the FastScanner. In this example we located a single step and
imaged that. This shows the step height as ~6A
Sample Images: MoS2 PFT Roughness
FastScanner
Looking at the
roughness over the
region that the area
was plane fit we get a
value of just 14pm Ra.
Sample Images: MoS2 TAP Step Height
FastScanner
Looking in tapping mode we again found a single step.
Sample Images: MoS2 TAP Roughness
FastScanner
Taking the roughness
measurement at this
location we get 15pm
Ra.
Instrument Noise Summary
From the four values we got on the day in PFT and TAP mode on both FastScanner and Icon Scanner:
Ra: 16pm, 23pm, 14pm, 15pm…
This is exceptional performance and we really didn’t have to try very hard. We could spend extra time
making everything as “quiet as possible” and get these numbers a bit lower but these serve as a good
example of what the instrument can produce in terms of low noise. I would add as well – these numbers are
great for a sample scanning system…the fact we can do it with a tip scanning system with such a huge
mechanical loop between tip and sample allowing for sample flexibility shows how sensitively engineered the
AFM + acoustic enclosure really is.
Ra 14pm
Flexibility
Performance
Speed Test on Customer Calibration
Grid
Tip Velocity 233um per second
Tip Velocity 80um per second
These 20um scan size images were taken with The FastScanner and Icon scanner respectively.
We optimized imaging such that we were just attaining “acceptable” image quality in terms of
tracking and gain noise etc…
Full Summary
In one line: We see the Icon as the most fully featured, high performance, stable, flexible, expandable,
robust AFM solution for multiuser environments.
Ra 14pm
12.Copyright
©
März 2016
Bruker Corporation. All rights reserved
23