SNC 2P Chemical & Physical Change Lab
advertisement

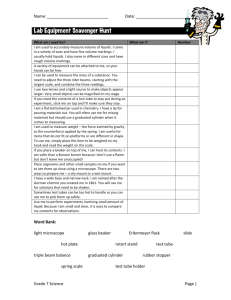
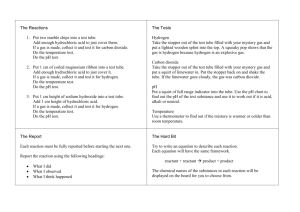
SNC 2P: Grade 10 Applied Science Lab Exercise: Identifying Physical and Chemical Changes Total /20 Name: __________________________________☺ A chemical property describes the ability of a substance to react to form a NEW substance. When this happens, we know that a chemical change has occurred. In a physical change, NO new substance is created. Learning Goal: To collect and use evidence from personal observations to identify and explain physical and chemical changes. Equipment and Materials: ○ Eye protection ○ Lab Apron ○ 2 test tubes ○ Test tube rack ○ Test tube stopper ○ Laboratory scoop ○ Graduated cylinder ○ Dropper bottles of dilute hydrochloric acid (HCl) and ○ dilute sodium hydroxide (NaOH) ○ 2 pieces of magnesium ribbon (Mg) ○ Steel wool ○ Wooden splint ○ Copper (II) sulphate (CuSO4) Change 1: 1. Use a graduated cylinder to add 4 mL of hydrochloric acid (HCl) to a clean test tube. 2. Add two 1 cm long strips of magnesium ribbon to the test tube. 3. Check for evidence of change occurring. Test the bottom of the test tube with your hand for temperature changes. 4. Put a stopper on the test tube to collect any gas that may be forming. 5. Record your observations in Table 1. Use as much scientific vocabulary as possible. Change 2: 1. 2. 3. 4. Light a splint. Hold the burning splint near the mouth of the test tube. Record your observations in Table 1. Give the remaining contents of the test tube to your teacher. Change 3: 1. 2. 3. 4. Use a graduated cylinder to add 5 mL of water into a graduated cylinder. Add about 0.5 g of copper (II) sulphate to the test tube. This is just the tip of the spatula. Stopper and invert the test tube several times to mix its contents well. Record your observations in Table 1. Change 4: 1. 2. 3. 4. Remove the stopper. Add a small ball of steel wool (the size of a small pea) the test tube. Stopper the test tube again. Wait 3 minutes and record the colour of the deposit on the steel wool. Change 5: 1. Remove the stopper. Add about 5 drops of sodium hydroxide (NaOH). 2. Record your observations in Table 1. Use as much chemistry vocabulary as possible. 3. Dispose of the contents of the test tube down the sink with lots of water. Clean your glassware and return all of your materials to where you picked them up. 5 marks available for observations recorded in Table 1. Table1: Observations and Conclusions: Chemical or Physical Change? Change Description 1. Hydrochloric acid (HCl) + magnesium (Mg) 2. Observations a.Chemical or Physical change? b. Explain how you know. a. b. a. Burning splint + mystery gas b. 3. a. Copper (II) sulphate + water b. 4. a. Copper (II) sulphate solution + steel wool 5. b. a. Mixture from Step 4 + sodium hydroxide (NaOH) b. Analyze and Evaluate: 1. Classify each of the 5 changes that you observed as either chemical or physical. Use specific evidence from your observation table to explain your choice. Include this information in Table 1. (10 marks) For example: I know that change X is a chemical change because heat was produced and heat is a sign of a chemical change. Use your notes to remind yourself of the signs of chemical vs physical changes. 2. a. Give one example from your everyday life of a physical change that is reversible. Explain. (2 marks) b. Give an example of a physical change that is NOT reversible. Explain. (2 marks) 3. In Change 2, you may have heard a “pop” when the burning splint was inserted into the mouth of the test tube. Look at the following chemical equation that represents the reaction in Change 1. Name the gas produced in the test tube. (1 mark) Mg + 2 HCl → H2 +MgCl2