Reinforcement Revision worksheets
advertisement

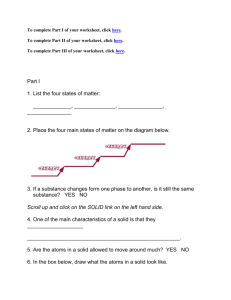
Towheed International School Name:__________ Reinforcement Grade: Six, Section: ( ) Revision worksheets Skills Worksheet It’s All Mixed Up Complete this worksheet after you finish reading the section “Mixtures.” Label each figure below with the type of substance it BEST models: colloid, compound, element, solution, or suspension. Reinforcement continued 6. Why did you label the figures on the previous page as you did? ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ PROFESSOR JUMBLE’S CONFUSION In her lab, Professor Jumble has four shelves labeled “Suspensions,” “Solutions,” “Compounds,” and “Colloids,” respectively. Last night, the professor set one beaker of clear liquid on each of the four shelves. When the professor walked into her lab this morning, all four beakers were on the same shelf, and she didn’t know which was which. She tested each beaker, and the results are below. 7. Use the test results to help Professor Jumble unjumble the beakers, and write the identity of each liquid in the blanks. Beaker A: ______________________ Beaker C: ______________________ • Light passes right through. • Liquid scatters light. • Particles do not separate in a • Liquid centrifuged into two different- centrifuge or a filter. • Upon heating, the liquid evaporates, and a crystal powder remains. colored layers. • Particles were left behind in the filter. Beaker B: ______________________ Beaker D: ______________________ • Light passes right through. • Liquid scatters light. • Particles do not separate in a • Liquid passes through a filter without centrifuge or a filter. • Upon heating, the liquid evaporates, but no residue remains. • The particles could not be separated by any other physical changes. leaving a residue. Use the terms from the following list to complete the sentences below. Each term may be used only once. Some terms may not be used. solvent solution compound solute mixture metalloids alloys suspensions 1. A pure substance made of two or more elements that are chemically combined is called a(n) ______________________ 2. If a spoonful of salt is mixed in a glass of water, the salt is the ______________________ 3. Solid solutions of metals or nonmetals dissolved in metals are called ______________________ 4. A colloid has the properties of solutions and______________________ 5. Particles of two or more substances that are distributed evenly among each other form a(n).______________________ Valerie placed 1.0 g of salt into one beaker, 1.0 g of soil into a second beaker, and 1.0 g of sugar into a third beaker. She then added 200 mL of water to each beaker and stirred the contents for 3 minutes. How many solutions did Valerie make? Identify the solvent and solute of each of the solutions. ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ ______________________________________________________________ CONCEPT MAPPING . Use the following terms to complete the concept map below: pure substances physical or chemical chemical solutions suspensions compounds States of Matter USING KEY TERMS Use the terms from the following list to complete the sentences below. Each term may be used only once. Some terms may not be used. Charles’s law evaporation sublimation condensation gas viscosity endothermic liquid Boyle’s law 1. The drops of water that appear on the outside of a glass of cold juice on a warm day are an example of ______________________. 2. The way a balloon decreases in volume when the temperature is decreased illustrates ______________________. 3. The change of state from a liquid to a gas is ______________________. 4. Sublimation is a change of state from a solid directly to a(n) ______________________. 5. In a(n) ______________________ change, energy is added to a substance. 6. One property of liquids is ______________________. Introduction to Electricity USING KEY TERMS Use the terms from the following list to complete the sentences below. Each term may be used only once. Some terms may not be used. electric current electric power static electricity series circuit electrical electrical insulators voltage 1. Plastic, glass, wood, and air are examples of good .______________________ 2. Electrons moving in a wire make up ______________________ and provide energy to the things that you use each day. 3. Burglar alarms are best wired using a(n) ______________________. 4. When the voltage is in volts and the current is in amperes, ______________________ is expressed in watts. 5. When your clothes come out of the dryer stuck together, they are full of ______________________. Skills Worksheet Reinforcement Make a State-ment Complete this worksheet after you finish reading the section “Behavior of Gases.” Each figure below shows a container that is meant to hold one state of matter. Identify the state of matter, and write the state on the line below the corresponding figure. Then write each of the descriptions listed below in the correct boxes. Some descriptions may go in more than one box. Particles are close together. Particles are held tightly in place by other particles. Particles break away completely from one another. changes volume to fill its container changes shape when placed in a different container State of matter has viscosity obeys Boyle’s law amount of empty space can change has definite shape Particles vibrate in place. does not change in volume has surface tension Description Name ______________________________ Class ___________________ Date __________________ Skills Worksheet Directed Reading B Section: Changes of State ENERGY AND CHANGES OF STATE Draw a line from each term to the matching number on the picture. freezing evaporation condensation melting MELTING: SOLID TO LIQUID Adding Energy Read the words in the box. Read the sentences. Fill in each blank with the word or phrase that best completes the sentence. melting endothermic 5. A change of state from a solid to a liquid is called______________________ 6. When energy is gained by something during a change of state, a(n)______________________ change occurs. Name ______________________________ Class ___________________ Date __________________ Directed Reading B continued FREEZING: LIQUID TO SOLID Removing Energy exothermic freezing 7. The melting point of a substance is the same as its______________________ point. 8. When energy is removed from a substance, a(n)______________________ change may occur. EVAPORATION: LIQUID TO GAS Boiling and Evaporation change of state evaporation 9. The particles break away from each other during ______________________. 10. When water boils, a(n)______________________ occurs. Effects of Pressure on Boiling Point Circle the letter of the best answer for each question. 11. What happens to the boiling point of a substance when you go higher above sea level? a. The boiling point gets higher. b. The boiling point stays the same. c. The boiling point gets lower. d. The substance won’t boil. Name ______________________________ Class ___________________ Date __________________ Directed Reading B continued CONDENSATION: GAS TO LIQUID Circle the letter of the best answer for each question. 12. What is condensation? a. the change of state from a liquid to a gas b. the change of state from a solid to a gas c. the change of state from a gas to a solid d. the change of state from a gas to a liquid SUBLIMATION: SOLID TO GAS Read the words in the box. Read the sentences. Fill in each blank with the word or phrase that best completes the sentence. endothermic sublimation 13. Dry ice changes from a solid to a gas during ______________________. 14. Sublimation is a(n)______________________ change. CHANGE OF TEMPERATURE VS. CHANGE OF STATE Circle the letter of the best answer for each question. 15. What happens when the temperature of a substance changes? a. The speed of the particles stays the same. b. The speed of the particles changes. c. The substance always melts. d. The substance always freezes. Name ______________________________ Class ___________________ Date __________________ Skills Worksheet Reinforcement Charge! Complete this worksheet after you have finished reading the section “Electric Charge and Static Electricity.” There are three ways for an object to gain a charge: friction, conduction, and induction. When it loses its charge, it experiences electric discharge. Label the following pictures as examples of conduction, induction, friction, or electric discharge