Naming PP
advertisement

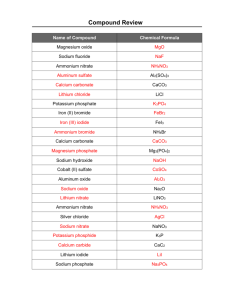
Nomenclature Table of Contents ‘Nomenclature’ Binary Compounds - Metal (fixed oxidation) + Nonmetal Criss-Cross Rule Binary Compounds - Metal (variable oxidation) + Nonmetal Binary Compounds - Nonmetal + Nonmetal Ternary Compounds Binary Hydrogen Compounds Meaning of Suffixes Empirical Formula Subscripts, Superscripts, and Coefficients Centrum Multivitamin Polyatomic Ions Contain only more than two types elements of elements We willwill These cover nottwo these be types covered in aof separate unit Four Types of Naming • Binary compounds • • Ternary compounds • • Coordination compounds • Organic compounds Binary Compounds Metals (fixed oxidation) + Nonmetals Examples: Binary Compounds Binary compounds that contain a metal of fixed oxidation number (group 1, group 2, Al, Zn, Ag, etc.), and a non-metal. To name these compounds, give the name of metal followed by the name of the non-metal, with the ending replaced by the suffix –ide. NaCl sodium chloride (Na1+ Cl1-) CaS calcium sulfide (Ca2+ S2-) AlI3 aluminum iodide (Al3+ I1-) Cations and Anions Common Simple Cations and Anions Cation H 1+ Li 1+ Na 1+ K 1+ Cs 1+ Be 2+ Mg 2+ Al 3+ Ag 1+ Name hydrogen lithium sodium potassium cesium beryllium magnesium aluminum silver Anion H 1F 1Cl 1Br 1I 1- iodide O 2S 2- Name* hydride fluoride chloride bromide oxide sulfide *The root is given in color. Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 86 Criss-Cross Rule Criss-Cross Rule Example: Aluminum Chloride Step 1: Aluminum Chloride Step 2: Al3+ Cl1- Step 3: Al 1 Cl 3 Step 4: AlCl 3 Criss-Cross Rule Example: Aluminum Oxide Step 1: Aluminum Oxide Step 2: Al3+ O2- Step 3: Al 2 O3 Step 4: Al2O3 Criss-Cross Rule Example: Magnesium Oxide Step 1: Magnesium Step 2: Mg2+ O2- Step 3: Mg 2 O2 Step 4: Step 5: Mg2O2 MgO Oxide Naming Binary Compounds Formula 1 BaO 2 NaBr 3 ________________ 4 5 MgI2 6 7 KCl 8 9 ________________ SrF2 10 11 ________________ CsF Name barium oxide ____________________ sodium bromide ____________________ magnesium iodide ____________________ potassium chloride strontium fluoride cesium fluoride Hungry for Tater Tots? Binary Compounds Metals (variable oxidation) + Nonmetals Binary Compounds Containing a Metal of Variable Oxidation Number To name these compounds, give the name of the metal (Type II cations) followed by Roman numerals in parentheses to indicate the oxidation number of the metal, followed by the name of the nonmetal, with its ending replaced by the suffix –ide. Examples Stock System Traditional System FeCl3 Iron (III) chloride Ferric chloride FeCl2 Iron (II) chloride Ferrous chloride SnO SnO2 Tin (II) oxide Tin (IV) oxide (“ic” ending = higher oxidation state; Stannous oxide Stannic oxide “ous” is lower oxidation state) Type II Cations Common Type II Cations Ion Stock System Fe 3+ Fe 2+ Cu 2+ Cu 1+ Co 3+ Co 2+ Sn 4+ Sn 2+ Pb 4+ Pb 2+ Hg 2+ Hg2 2+ iron (III) iron (II) copper (II) copper (I) cobalt (III) cobalt (II) tin (IV) tin (II) lead (IV) lead (II) mercury (II) mercury (I) Traditional System ferric ferrous cupric cuprous cobaltic cobaltous stannic stannous plumbic plumbous mercuric mercurous *Mercury (I) ions are always bound together in pairs to form Hg2 2+ Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 90 PbO2 Naming Binary Compounds Formula 1 Hg2O 2 3 HgO 4 CuF2 5 ________________ 6 7 ________________ Cu2S 8 9 Cr2O3 10 11 ________________ Name ____________________ mercury (II) oxide ____________________ mercury (I) oxide copper (II) fluoride copper (I) sulfide chromium (III) oxide ____________________ lead (IV) oxide Binary Compounds Nonmetal + Nonmetal Prefixes you should know: Binary Compounds Containing Two Nonmetals To name these compounds, give the name of the less electronegative element first with the Greek prefix indicating the number of atoms of that element present, followed by the name of the more electronegative nonmetal with the Greek prefix indicating the number of atoms of that element present and with its ending replaced by the suffix –ide. Mono 1 Di 2 Tri 3 Tetra 4 Penta 5 Hexa 6 Hepta 7 Octa 8 Nona 9 Deca 10 Binary Compounds Containing Two Nonmetals (Type III Compounds) As2S3 1. ________________ 2. SO2 3. ________________ 4. 5. P2O5 6. CO2 7. ________________ 8. 9. N2O5 10. 11. H2O diarsenic trisulfide sulfur dioxide diphosphorus pentoxide ____________________ carbon dioxide dinitrogen pentoxide ____________________ dihydrogen monoxide ____________________ Naming Binary Compounds Binary Compound? Yes Metal Present? No Type III Use Greek Prefixes Yes Does the metal form more than one cation? No Type I Use the element name for the cation. Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 98 Yes Type II Determine the charge of the cation; use a Roman numeral after the cation name. Ternary Compounds Ternary Compounds Ternary compounds are those containing three different elements. (NaNO3, NH4Cl, etc.). The naming of ternary compounds involves the memorization of several positive and negative polyatomic ions, (two or more atoms per ion), and adding these names to the element with which they combine. i.e., Sodium ion, Na1+ added to the nitrate ion, NO31-, to give the compound, NaNO3, sodium nitrate. Binary rules for indicating the oxidation number of metals and for indicating the numbers of atoms present are followed. The polyatomic ions that should be learned are listed in a separate handout. Ternary Compounds NaNO2 KClO3 Ca3(PO4)2 Fe(OH)3 NaHCO3 sodium nitrite potassium chlorate calcium phosphate iron (III) hydroxide sodium bicarbonate ‘sodium hydrogen carbonate’ Common Polyatomic Ions Names of Common Polyatomic Ions Ion NH4 1+ NO2 1NO3 1SO3 2SO4 2HSO4 1OH 1CN 1PO4 3HPO4 2H2PO4 1- Name ammonium nitrite nitrate sulfite sulfate hydrogen sulfate (“bisulfate” is a widely used common name) hydroxide cyanide phosphate hydrogen phosphate dihydrogen phosphate Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 100 Ion CO3 2HCO3 1ClO 1ClO2 1ClO3 1ClO4 1C2H3O2 2MnO4 1Cr2O7 2CrO4 2O2 2- Name carbonate hydrogen carbonate (“bicarbonate” is a widely used common name) hypochlorite chlorite chlorate perchlorate acetate permanganate dichromate chromate peroxide Ternary Compounds Ca3(PO4) 2 1. ________________ 2. (NH4)2CO3 3. ________________ 4. Al2(SO4)3 5. ________________ 6. 7. Na2SO4 8. LiCN 9. 10. Ba(ClO3)2 11. 12. ________________ Cu(OH)2 calcium phosphate ammonium carbonate aluminum sulfate sodium sulfate ____________________ lithium cyanide ____________________ ____________________ barium chlorate copper (II) hydroxide Magnesium Phosphate Step 1: Magnesium Step 2: Mg2+ PO43- Step 3: Mg 3 (PO4) 2 Step 4: Phosphate Mg3(PO4)2 Naming Chemical Compounds Binary Compound? No Polyatomic ions present? No This is a compound for which naming procedures have not yet been considered. Zumdahl, Zumdahl, DeCoste, World of Chemistry 2002, page 102 Yes Use the strategy summarized earlier Yes Name the compound using procedures similar to those for naming binary ionic compounds. Binary Hydrogen Compounds Oxysalts + H2O Oxyacids Binary Hydrogen Compounds of Nonmetals When Dissolved in Water (These compounds are commonly called acids.) The prefix hydro- is used to represent hydrogen, followed by the name of the nonmetal with its ending replaced by the suffix –ic and the word Acid added. Examples: *HCl HBr Hydrochloric acid Hydrobromic acid *The name of this compound would be hydrogen chloride if it was NOT dissolved in water. Naming Simple Chemical Compounds Ionic (metal and nonmetal) Metal Forms only one positive ion Use the name of element Forms more than one positive ion Use element name followed by a Roman numeral to show the charge Covalent (2 nonmetals) Nonmetal Single Negative Ion Use the name of the element, but end with ide First nonmetal Second nonmetal Before element name use a prefix to match subscript Use a prefix before element name and end with ide Polyatomic Ion Use the name of polyatomic ion (ate or Ite) Naming Ternary Compounds from Oxyacids The following table lists the most common families of oxy acids. one more oxygen atom HClO4 perchloric acid most “common” HClO3 chloric acid H2SO4 sulfuric acid H3PO4 phosphoric acid HNO3 nitric acid one less oxygen HClO2 chlorous acid H2SO3 sulfurous acid H3PO3 phosphorous acid HNO2 nitrous acid two less oxygen HClO hypochlorous acid H3PO2 hypophosphorous acid (HNO)2 Hyponitrous acid Oxyacids Oxysalts If you replace hydrogen with a metal, you have formed an oxysalt. A salt is a compound consisting of a metal and a non-metal. If the salt consists of a metal, a nonmetal, and oxygen it is called an oxysalt. NaClO4, sodium perchlorate, is an oxysalt. OXYACID OXYSALT HClO4 perchloric acid NaClO4 sodium perchlorate HClO3 chloric acid NaClO3 Sodium chlorate HClO2 chlorous acid NaClO2 Sodium chlorite HClO hypochlorous acid NaClO Sodium hypochlorite ACID SALT per stem ic changes to per stem ate stem ic changes to stem ate stem ous changes to stem ite hyper stem ous changes to hypo stem ite HClO3 acid + Na1+ cation NaClO3 + H1+ salt Suffixes have meaning “-ide” binary compound sodium chloride (NaCl) “-ite” or “-ate” sulfite (SO32-) sulfate (SO42-) “-ol” polyatomic compound “-ate” means one more oxygen than “-ite” alcohol methyl alcohol (methanol) “-ose” sugar sucrose “-ase” sucrase enzyme Prefixes – Binary Molecular Compounds Greek Prefixes for Two Nonmetals Number Indicated 1 2 3 4 5 6 7 8 9 10 Prefixes mono di tri tetra penta hexa hepta octa nona deca Binary Molecular Compounds N2O N2O3 N2O5 ICl ICl3 SO2 SO3 dinitrogen monoxide dinitrogen trioxide dinitrogen pentoxide iodine monochloride iodine trichloride sulfur dioxide sulfur trioxide Oxidation States in Formulas and Names Traditional System Stock System +1 dinitrogen monoxide dinitrogen trioxide dinitrogen pentoxide sulfur dioxide sulfur trioxide -2 N2O N +3 2O -2 3 N2O5 SO +5 -2 2 SO3 +4 -2 +6 -2 nitrogen (I) oxide nitrogen (III) oxide nitrogen (V) oxide sulfur (IV) oxide sulfur (VI) oxide Subscripts, Superscripts and Coefficients Chemical Formulas C8H18 Subscript indicates that there are 8 carbon atoms in a molecule of octane. Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 203 Subscript indicates that there are 18 hydrogen atoms In a molecule of octane. Stock System of Nomenclature CuCl2 Name of cation + Roman Name of anion numeral indicating charge copper (II) chloride Chemical Formulas Al2(SO4)3 Subscript 2 Subscript 4 Subscript 3 refers to refers to refers to everything inside parentheses. 2 aluminum atoms. 4 oxygen Here there are 3 sulfate ions, atoms in sulfate ion. with a total of 3 sulfur atoms and 12 oxygen atoms. Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 204 The Stock System of Nomenclature CuCl2 Name of cation + Roman numeral indicating charge Copper (II) Davis, Metcalfe, Williams, Castka, Modern Chemistry, 1999, page 208 Name of anion chloride Centrum Multivitamin Centrum Multi-Vitamin Ingredients: ascorbic acid, beta carotene, biotin, calcium pantothenate, calcium phosphate, carnauba wax, chromium chloride, crospovidone, cupric sulfate, cyanocobalamin, dl-alpha tocopheryl acetate, FD & C blue no. 2 aluminum lake, hydroxypropyl cellulose, hydroxypropyl methylcellulose, lactose, magnesium oxide, magnesium stearate, manganese sulfate, microcrystalline cellulose, niacinamide, nickel sulfate, phytonandione, polyethylene glycol, potassium chloride, potassium citrate, potassium iodide, povidone, pyridoxine hydrochloride, riboflavin, silica gel, sodium borate, sodium metavanadate, sodium molybdate, sodium selenate, stannous chloride, stearic acid, thiamin mononitrate, titanium dioxide, triacetin, vitamin A acetate, vitamin D3, zincproducts oxide. is a leading PC7563-46-00 Warning: Accidental overdose of iron-containing cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control immediately. Chromium (III) Chloride RECALL: Chromium forms oxides in which metal exhibits oxidation states of +3 and +2. STOCK system indicates oxidation state of compound. Assume Cr3+ (chromium (III) chloride). Step 1: Chromium (III) Step 2: Cr3+ Cl1- Step 3: Cr 1 Cl 3 Step 4: Chloride CrCl3 Return to Centrum Bottle Cupric Sulfate RECALL: “ic” higher oxidation & Cu2+ (higher) “ous” lower oxidation Cu1+ (lower) Step 1: Cupric Sulfate Step 2: Cu2+ SO42- Step 3: Cu Step 4: Step 5: 2 (SO4) 2 Cu2(SO4)2 CuSO4 Return to Centrum Bottle Manganese (III) Sulfate RECALL: Manganese forms oxides in which metal exhibits oxidation states of +2, +3, +4, and +7. STOCK system indicates oxidation state of compound. Assume Mn3+ (manganese (III) sulfate). Step 1: Manganese (III) Sulfate Step 2: Mn3+ SO42- Step 3: Mn 2 (SO4) 3 Step 4: Mn2(SO4)3 Return to Centrum Bottle Stannous Chloride RECALL: “ic” higher oxidation & Sn4+ (higher) Step 1: “ous” lower oxidation Sn2+ (lower) Stannous (tin) Chloride Step 2: Sn2+ Cl1- Step 3: Sn 1 Cl 2 Step 4: SnCl2 Return to Centrum Bottle Stannic Chloride RECALL: “ic” higher oxidation & Sn4+ (higher) “ous” lower oxidation Sn2+ (lower) Step 1: Stannic (tin) Chloride Step 2: Sn4+ Cl1- Step 3: Sn 1 Cl 4 Step 4: SnCl4 Return to Centrum Bottle Chromium Chloride RECALL: Chromium has multiple oxidation states. Name with STOCK system. Assume Chromiun (II). Step 1: Chromium (II) Step 2: Cr2+ Cl1- Step 3: Cr 1 Cl 2 Step 4: Cr1Cl2 Step 5: CrCl2 Chloride Return to Centrum Bottle Calcium Phosphate Step 1: Calcium Phosphate Step 2: Ca2+ PO43- Step 3: Ca Step 4: 3 (PO4) 2 Ca3(PO4)2 Return to Centrum Bottle Zinc Oxide Step 1: Zinc Oxide Step 2: Zn2+ O2- Step 3: Zn O2 Step 4: Step 5: 2 Zn2O2 ZnO Return to Centrum Bottle Polyatomic Ions Common Polyatomic Ions