A14
advertisement

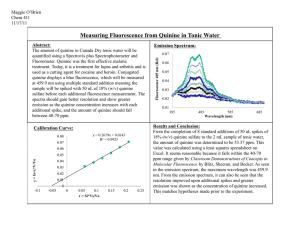
A14 Alkaloids Alkaloids are a diverse group of secondary metabolites found in many organisms. These organic compounds, most of which are derived from plants, are created naturally through various biosynthetic pathways and have different chemical structures. Although alkaloids are chemically different, they all share a basic definition. Alkaloids are commonly classified as a cyclic organic compounds containing nitrogen in a negative oxidation state, often with the nitrogen contained within a heterocycle8. They are also recognized as naturally occurring substances that are not vital to the organism that produces them. A vast array of chemical differences allow alkaloids to produce many neurological and physiological effects to the organism that consumes them. These effects naturally gave rise to the popularity of alkaloid-base plants among humans for both good and bad uses. Alkaloid use dates back to early civilization, varying in use from important medicines to even poisons. One of the most famous instances of alkaloid use was in the death of the famous philosopher Socrates through the consumption of coniinecontaining hemlock5. Coniine (2-propylpiperidine) works by antagonistically binding to the nicotinic receptor which causes muscle paralysis. This paralysis eventually leads to respiratory failure, which then leads to death due to the lack of oxygen supply to the brain. In 1886, Albert Ladenburg was able to synthesize coniine in the lab through the chemical reaction depicted below. This reaction however, was by no means efficient and was later improved. Another alkaloid, atropine, was made famous by Cleopatra. Atropine was found in henbane and was used to dilate Cleopatra’s eyes to make her appear more alluring6. In modern times, chemical derivatives of atropine are used during eye exams to dilate the eye during observation. Even though alkaloids have been used for centuries, the first alkaloid was not identified until 1806 when Serturner isolated morphine from the opium poppy, Papaver somniferum9. Since its discovery, morphine has grown to be an important analgesic drug for managing severe pain and suffering in patients. It is most commonly used in surgical procedures in conjunction with anesthesia. Furthermore, it is also a drug of choice for treating those who are terminally ill or those who are suffering from cancer. Aside from providing pain relief, morphine was also known to cause a sense of euphoria. This is the reason for its original popularity during the 19th century and even today. However, its modern-day popularity can be attributed to the street drug heroin. Heroin was first synthesized by C.R. Alder Wright in 1874, when he decided to add acetyl groups to the molecular structure of morphine in the synthetic process illustrated below4. Wright’s creation of the drug diacetylmorphine did not lead to further development and the drug was not popularized until a new synthesis pathway was discovered by Felix Hoffman. Hoffman determined that the acetylated morphine was fast acting and was about two times more potent than morphine itself. Bayer Pharmaceutical products eventually released the drug under the name Heroin, claiming it was a non-addictive morphine substitute4. It was later found that heroin was a quicker-acting form of morphine and was also broken down readily into morphine as well. Because of its high potency and notoriety for inducing severe drug dependence, heroin has been made illegal in the United States. Even with its ban, people still find a way to buy heroine in its impure form, causing a major health risk in the United States. The most common risk associated with heroin use is the spread of blood-borne illnesses through the sharing of hypodermic needles. Aside from heroin, morphine also has many other derivatives such as hydrocodone, oxycodone, and codeine, all of which provide relief in some manner with less potency and with less addictive properties. Another alkaloid of great importance in the field of medicine is the compound quinine. Quinine is found in the ground bark of cinchona trees and has been used as a treatment for malaria since the early 17th century. It proved to be a vital drug for Europeans in colonial times when malaria was rampant. Quinine allowed Europeans to colonize parts of Africa without succumbing to the deadly effects of malaria. It was also the “prime reason why Africa ceased to be known as the white man’s grave”10. The importance of quinine inspired scientists to find a way to chemically synthesize quinine but this never took root due to the high cost of production. Today, cinchona trees are still the only economically practical source of quinine. Recently, quinine has been considered a last resort in the treatment of malaria because of the unpleasant side effects it may cause. Quinine has readily been replaced by the drug chloroquine, but it is still used in poor countries where quinine is much cheaper and more easily available10. Aside from its use as an anti-malaria drug, quinine is also used as flavoring in drinks, most commonly tonic water, because it provides a slight bitter taste. Furthermore, quinine is used in photochemistry as a fluorescent standard due to its relatively constant fluorescent quantum yield (wiki). Its absorption reading peaks around 350 nm while its fluorescent emissions peak around 460 nm 10. When UV light is shone onto a solution that contains quinine, for instance tonic water, it glows a cyan blue. Early in the 20th century, cancer became an important topic of research in medicine. With little traction in finding a cure, one alkaloid created the first developmental stride in providing hope to the masses. Paclitaxel was discovered in 1967 by Monroe E. Wall and Mansukh C. Wani from the National Cancer Institute. They isolated the drug from the pacific yew tree, Taxus brevifolia, and named it Taxol6. Taxol is a mitotic inhibitor which works by stabilizing microtubules in the cell so that they are unable to break down during cell division. It is approved in the UK for the treatment of ovarian, breast, lung, and other cancers, but is very expensive because of its limited availability. This limited availability is due to scarcity of the resource it is harvested from. With such limited quantities, many are striving to synthetically produce the drug efficiently and easily. The challenge of synthesizing Taxol in the lab continues to be a very daunting task. While some alkaloids may save lives, another widely known alkaloid does the complete opposite. This alkaloid contributes to almost 500,000 deaths each year because of the dependency it induces in individuals. This alkaloid is nicotine, and it is found in the leaves of the plant tobacco. Nicotine contains two nitrogen rings, one of which is pyrrolidine and the other pyridine7. These chemical groups help nicotine bind to the nicotinic acetylcholine receptor and act as an agonist. This causes increased stimulation of the reward receptors in the brain, creating a sense of euphoria. This euphoric effect is one of the reasons why nicotine is so addictive and is sometimes compared in likeness to that of cocaine and heroin. This addictiveness leads to increased tobacco use, and it ultimately causes many negative effects on one’s health later in life. To stop patients form smoking tobacco, a nicotine replacement regiment can be implemented in which gum, patches, or lozenges are used to slowly eliminate nicotine dependency. Lastly, there is one alkaloid that is digested by millions every day. It is known to many as caffeine. Caffeine is found in various seeds, leaves, and fruit of some plants, more specifically from coffee beans and tea leaves. Caffeine acts as a psychoactive stimulant in the body. It is the most consumed drug in the world. Over 90% of all American adults consume caffeine on a daily basis. Once ingested, caffeine is metabolized in the liver by the cytochrome P450 oxidase enzyme into three different metabolites: paraxanthine, theobromine, and theophylline. Paraxanthine is the metabolite produced in greatest amounts. It causes elevated glycerol and fatty acid levels in the blood. Theobromine, the second most produced metabolite from caffeine, causes blood vessels to dilate and also causes an increased urine volume. The third and least abundant metabolite produced from caffeine is theophylline. It causes relaxation of smooth muscles in the bronchi and is commonly used as treatment for asthma. The chemical structure of caffeine is comprised of two fused rings, one being a pyrimidinedione and the other an imidazole. It is usually synthesized in plants from the purine nucleotides adenosine monophosphate (AMP), guanosine monophosphate (GMP), and inosine monophospate (IMP)3. With its close resemblance to adenosine, caffeine is able to block the adenosine receptors in the brain, stopping the suppression of activity in the central nervous system. In too large doses however, caffeine begins to inhibit the GABA receptor which results in insomnia, anxiety, and increased heart rate/respiration. With such variability in how they affect the human body, alkaloids have become an important part of medicinal research. This continuously growing library of plant constituents provides models for the synthesis of modern synthetic drugs. With the help of plant extract screening programs, new drugs are continually being discovered, in the hopes that one of those drugs may change the world one day. Work Cited 1. Achan, Jane, et al. "Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria." Malar J 10.144 (2011): 1475-2875. 2. "Alkaloid." Wikipedia. Wikimedia Foundation, 02 July 2014. Web. 09 Feb. 2014. 3. "Caffeine." Wikipedia. Wikimedia Foundation, 02 Apr. 2014. Web. 06 Feb. 2014. 4. "Heroin." Wikipedia. Wikimedia Foundation, Web. 09 Feb. 2014. 5. Kutchan, Toni M. "Alkaloid Biosynthesis -The Basis for Metabolic Engineering of Medicinal Plants." The Plant Cell 7.7 (1995): 1059. 6. Nicolaou, K. C., et al. "Total synthesis of taxol." Nature 367.6464 (1994): 630-634. 7. "Nicotine." Wikipedia. Wikimedia Foundation, Web. 09 Feb. 2014. 8. Pelletier, S. "The Nature and Definition of an Alkaloid." Toxocology. N.p.: n.p., n.d. 27-31. Web. 09 Feb. 2014. 9. Roberts, Margaret F., and Michael Wink, eds. Alkaloids: biochemistry, ecology, and medicinal applications. Springer, 1998. 10. "Quinine." Wikipedia. Wikimedia Foundation, 02 July 2014. Web. 09 Feb. 2014.