1056391Notes 8.2
advertisement

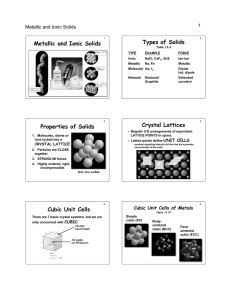
Forming Ionic Bonds • Electron transfer: one atom loses electrons - another atom gains those electrons • Positive and negative ions attract = ionic compound. • Metal + oxygen = OXIDE • Metal + nonmetals = SALT Ionic Bonds • Often binary (2 elements) • e- lost = e- gained (always) • Quick check: – How will sodium & chlorine combine? – How about calcium & sulfur? – How about lithium & phosphorus? – How about iron (III) & oxygen? Hint: use e- configurations or electron dot diagrams Then show box and table method to solve Quick Check – How will sodium & chlorine combine? calcium & sulfur? lithium & phosphorus? iron (III) & oxygen? Properties of Ionic Compounds • Crystals (not molecules) = many cations and anions packed into a repeating pattern (crystal lattice). • Chemical formula = ratio of atoms needed • Fairly strong bond = large amount of energy to break. • High melting & boiling points • Often rigid & hard More Properties • If a liquid or solution, good conductors of electricity (electrolytes) • If a solid, bad conductors because ions aren’t free to move • EXOTHERMIC (almost always) – means the ionic compound is more stable & requires less energy, so heat is given off when forming Ex: hand warmers = iron + oxygen combining Lattice Energy • • • • Energy to break apart 1 mole of ions Equals energy released when ions form More negative number = stronger bonds Smaller ions = greater lattice energy (nucleus is closer to the valence e-) • Greater ionic charge = greater lattice NRG Quick Check • Which would have the greater lattice energy? – LiCl or LiBr – KF or RbF – NaCl or MgF2 – SrCl2 or MgO • Which compound in each pair would have the higher melting point?