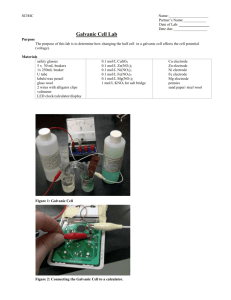
Chemische Grundbegriffe
advertisement

Electrochemistry Prof. Dr. Sabine Prys http://www.iccb.org/student/mod/science/mod_chem1/mod1/p1.html @designed by ps 3.3 Normal & Standard Conditions Such definitions can vary according to different sources: IUPAC, NIST, … normal conditions: normal pressure p = 1 atm = 101,325 kPa = 1013,25 mbar normal temperature T = 0°C = 273.15 K standard conditions: standard pressure p = 1 atm = 101,325 kPa = 1013,25 mbar standard temperature T = 25°C = 298.15 K 3.4 Enthalpy • Enthalpy is a measure of the total energy of a thermodynamic system including – the internal energy (energy required to create a system), – the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure. • Enthalpy is a thermodynamic potential, a state function and an extensive quantity (i.e. depending on amount material). H U p V H P enthalpy pressure U V internal energy volume http://goldbook.iupac.org 3.8 GIBBS’ Free Energy G Useful energy, or energy available to do work G G H T S U p V T S G U S V = free energy = internal energy = entropy = volume H T p = GIBBS’ energy (enthalpy) = Kelvin temperature = pressure TDS is the energy not available for doing work 3.9 Spontaneity of Redox Reactions G H T S DH DS Spontaneity Exothermic DH < 0 Increase DS > 0 + Exothermic DH < 0 Decrease DS < 0 + if |TDS| < |DH| Endothermic DH > 0 Increase DS > 0 + if TDS > DH Decrease DS < 0 - Endothermic DH > 0 DG < 0 DG > 0 3.10 Thermodynamical Equilibrium • Reversible processes ultimately reach a point where the rates in both directions are identical, so that the system gives the appearance of having a static composition at which the Gibbs energy G is a minimum DG = 0 • At equilibrium the sum of the chemical potentials of the reactants equals that of the products, so that: DG = DG298 + RT . lnK = 0 DG298 = - RT . lnK • The equilibrium constant K is given by the mass-law effect. http://goldbook.iupac.org 3.12 Maximum Work Wmax DG RT ln K z n F E U I t Wmax G R T K z n F E U I t = = = = = = = = = = = = maximum work Gibbs free energy gas constant Kelvin temperature equilibrium constant ion charge moles Faraday‘s constant galvanic cell potential voltage currant time 4.0 Chemical Solutions • Suspension • Colloid • Solution (particle diameters 10-4 - 10-5 cm ) solid particles in homogeneous fluid (particle diameters 10-5 - 10-7 cm ) microscopically dispersed particles in another substance (particle diameters 10-7 - 10-8 cm ) Homogeneous phase with at least 2 components: solvent and solute » gas in liquid e.g. O2 in H2O » gas in solid e.g. H2 in palladium » liquid in liquid e.g. petroleum » solid in liquid e.g. NaCl in H2O » electrolytes in water 4.6 Ion activity High ion concentrations in aqueous solutions ion – ion interactions: pH measured < pH calculated (1m, 0.1 m solution of acids) ion activity: a f c a = activity, f = activity coefficient, c = concentration f (HCl, 25°C): 0.001m/0.965 0.01m/0.905 0.1m/0.794 1m/0.809 4.7 Colloidal Solutions • Larger particles in solvent, e.g. macromolecules / polymers • Properties depend on solute size and not on solute concentration ! • Coagulation: growth of larger particles by smaller particles consumption • Hydrophobe colloids: large surface, large adsorption properties • Hydrophile colloids 4.9 Electrolytes Electrolyte: solution which conducts electrical current HClaq H aq Claq CH 3COOH aq H H 2O Hydrated H3O+ Hydrated OH- H 3O 3H 2O OH 3H 2O H aq CH 3COOaq H 3O H 9O4 H 7O4 4.9.1 Electrical Conductivity in Solutions Electrolytes • solutions which support ion transport • salts in aqueous solutions, e.g. KCl, ZnSO4, CuCl2, etc. • molten salts _ + H2O Conductivity L (resistance R) 1 L R + + + + + + 1 bad electrolyte: distilled water: 0.0548 µS/cm at 25 °C. cathode cat ions anode anions 4.9.2 Specific Conductivity L 1 1 R absolute electrolyte conductivity R = solution resistance l 1 1 A R m specific electrolyte conductivity A = electrode surface, l = electrode distance KCl concentration [mol / l] 0 1 0,1 0,01 0°C << 0,001 6,543 0,7154 0,07751 [1 / .m] 18°C << 0,001 9,820 1,1192 0,12227 25°C << 0,001 11,173 1,2886 0,14114 4.9.3 Example: Proton Migration Grotthuss Diffusion structural defect migration mesomeric structures between H9O4+ and H5O2+, 5.0 Electrochemical Cells - + H2 Cl2 + H2 Cl2 electrolytic cell galvanic cell 2 HCl (aq) H2 (g) + Cl2 (g) H2 (g) + Cl2 (g) 2 HCl (aq) electrical energy chemical energy chemical energy electrical energy 5.1 Electrolysis electrolysis: decomposing materials by electric current H2SO4 + 2H2O 2H3O+ + SO42electrods water electrolysis cathodic reduction 4H3O+ + 4e- 2 H2 + 2 H2O anodic oxidation 4 OH 2 H 2 O + O2 + 4 e total 2 H2O (l) 2 H2 (g) + O2 (g) battery ca. 15 V H20 + H2SO4 1:10 5.1.1 Electrochemical Equivalent Q F n M Ec zF F e NL Q = electric charge in C n = yield in mol F = Faraday‘s constant = 96485,309 As / mol Ec = electrochemical equivalent M = ion weight z ion charge = NL = Lohschmidt‘s number e elementary charge = 5.1.2 Faraday‘s Laws m = Ec . Q = Ec .I. t m = Ec = Q = I t = = mass yield in g electrochemical equivalent electric charges in Coulomb current strength electrolysis time ma M a zb mb z a Mb ma ,mb = mass yield in g for material a / b Ma,Mb = molecular weight for material a / b za, zb = chemical valency for material a / b 5.2 Galvanic Elements voltmeter ca 1,1 V Daniell Element: 2 galvanic half cells + bridge Zn / ZnSO4 // CuSO4 / Cu electrode reactions Zn (cathode) Zn2+ + 2eCu2+ + 2e Cu (anode) Zn 1m ZnSO4 Cu 1m CuSO4 diaphragma (pottery) Zn metal in ionic solution Cu ions in Cu metal electrical current results from different oxidation affinities bridge containing KCl solution 5.4 Standard Hydrogen Electrode standard hydrogen electrode (SHE) = reference potential = E0 = 0 V H2 2H+ + 2ep = 1,01325 bar T = 25°c a(H+) = 1 mol / l c(H+) = 1,235 mol / l (HCl) H2 gas Pt electrode 5.5 Metal Standard Potentials standard hydrogen electrode = reference potential E0 = 0 V metal electrode / metal salt solution at standard conditions = standard metal potential M Mz+ + ze- p = 1,01325 bar T = 25°c c(Mz+ ) = 1 mol / l H2 gas Pt electrode pH < 6 precipitation prevention 68 5.5.1 Metal Standard Potential Tables Red Ox z E0 [Volt] K K+ +1 - 2,93 Na Na+ +1 - 2,71 Zn Zn2+ +2 - 0,76 Fe Fe2+ +2 - 0,44 Pb Pb2+ +2 - 0,13 H2 2 H+ +2 0,00 Cu Cu2+ +2 + 0,34 Ag Ag+ +1 + 0,80 Au Au3+ +3 + 1,50 pH-dependant 5.5.2 Calvanic Corrosion Potential Chart Galvanic Corrosion Potential Chart K, Na, Mg, Al, Zn, Fe, Pb, Cu, Ag, Au cathode least noble corroded metals strong oxidation affinity negative oxidation potential anode most noble protected metals weak oxidation affinity positive oxidation potential passivation of Al, Mg, Mn, Cr alternative corrosion potential charts for industrial materials 5.6.3 Exercise: Gibbs Free Energy What happens if ΔG = 0 5.7 NERNST‘s Equation 1 electrode potential dependency on temperature and concentration R T [ox] EE0 ln zF [red ] E E0 R T z F [ ] = measured cell potential = standard reaction potential = gas constant ( 8,3145 J . mol-1 . K-1) = Kelvin temperature = charges = Faraday’s constant = concentration of oxidant / reductant in mol / l 5.7.1 NERNST’s Equation 2 1. type electrode ( = metal electrode in metal salt solution) [red] = const R T E E0 ln[ox] zF E E0 R T z F [ox] 0,05916 25C :E E0 ln[ox] z = measured cell potential = standard reaction potential = gas constant ( 8,3145 J . mol-1 . K-1) = Kelvin temperature = charges = Faraday’s constant = concentrationen of oxidant in mol / l 5.7.2 Exercise: Maximum Electrical Voltage 1. Calculate the maximum electrical voltage for the Daniell element when standard conditions ! Daniell Element: Cu/Cu++//Zn++/Zn Cu/Cu++/ +0,34 V Zn++/Zn/ S= + 1,1 V +0,76 V 2. Calculate the maximum electrical voltage for a galvanic cell with Ni/Ni++//Zn++/Zn when standard conditions ! Ni/Ni++// S= -0,23 V + 0,53 V Zn++/Zn/ +0,76 V 5.7.3 Exercise: Nernst Equation What is the electrode potential for a silver electrode at 0°C when the Ag+ concentration is 1 mol ? RT : R T ln[ox ] zF J K mol [V ] [V ] mol K A s E E0 J [V ] A s [V ] 0C : E0,8 8,314 273,15 ln[1] 1 96,5 103 E 0,8 0,024 0,824 [V ] 5.8 Ag / AgCl Electrode 2. type electrode = metal electrode in saturated metal salt solution = electrode with constant potential (no concentration changes) L [ Ag ] [Cl ]1,7 10 L const [ Ag ] const T = 25 °C: 1 m KCl E0 = + 0,220 V sat. KCl E0 = + 0,1958 V 10 mol 2 l2 Ag K+ Ag+ ClAgClsat 5.8.1 Concentration Cells • Cu(s) | Cu2+ (0.05 M) || Cu2+ (2.0 M) | Cu(s) • half cell reactions : oxidation: reduction: overall reaction: Cu(s) Cu2+ (2.0 M) + 2 e– Cu2+ (2.0 M) → Cu2+ (0.05 M) + 2 e– → Cu(s) → Cu2+ (0.05 M) • cell's emf : • E = E°- (0.05916\2) log [0,05/2] = 0.0474 V • E° = 0 , (electrodes and ions are the same in both half-cells) 5.15 Dry Cells moist electrolyte paste Leclanché's cell – anode is a zinc container surrounded by a thin layer of MnO2 – Cathode a carbon bar inserted on the cell's electrolyte – moist electrolyte paste NH4Cl + ZnCl2 mixed with starch Anode: Zn(s) → Zn2+(aq) + 2 e– Cathode: 2 NH4+(aq) + 2 MnO2(s) + 2 e– → Mn2O3(s) + 2 NH3(aq) + H2O(l) Overall reaction: Zn(s) + 2 NH4+(aq) + 2 MnO2(s) → Zn2+(aq) + Mn2O3(s) + 2 NH3(aq) + H2O(l) E = ~ 1.5 V 5.16 Zn Battery Graphics: http://en.wikipedia.org/wiki/File:Zincbattery.png 5.17 Mercury Battery amalgamated anode of mercury and zinc surrounded by a stronger alkaline electrolyte and a paste of ZnO and HgO Mercury battery half reactions are shown below: Anode: Zn(Hg) + 2 OH–(aq) → ZnO(s) + H2O(l) + 2 e– Cathode: HgO(s) + H2O(l) + 2 e– → Hg(l) + 2 OH–(aq) Overall reaction: Zn(Hg) + HgO(s) → ZnO(s) + Hg(l) no changes in the electrolyte's composition when working 1.35 V of direct current Not rechargeable Graphics: http://en.wikipedia.org/wiki/File:Mercurybattery.png 5.18 Lead-Acid battery six identical cells assembled in series (6 x 2V ) = 12 V lead anode lead dioxide cathode Electrolyte sulfuric acid Anode: Pb(s) + SO42–(aq) → PbSO4(s) + 2 e– Cathode: PbO2(s) + 4 H+(aq) + SO42–(aq) + 2 e– → PbSO4(s) + 2 H2O(l) Overall reaction: Pb(s) + PbO2(s) + 4 H+(aq) + 2 SO42–(aq) → 2 PbSO4(s) + 2 H2O(l) Rechargeable (external voltage electrolysis of the products) http://en.wikipedia.org/wiki/Lithium-ion_battery 5.19 Lithium rechargeable battery (1) Positive electrodes Electrode material Average potential difference LiCoO2 3.7 V LiMn2O4 4.0 V LiNiO2 3.5 V LiFePO4 3.3 V Li2FePO4F 3.6 V LiCo1/3Ni1/3Mn1/3O2 3.6 V Li(LiaNixMnyCoz)O2 4.2 V Negative electrodes Graphite (LiC6) 0.1-0.2 V Hard Carbon (LiC6) Titanate (Li4Ti5O12) 1-2 V Si (Li4.4Si)[27] 0.5-1 V Ge (Li4.4Ge)[28] 0.7-1.2 V Specific capacity 140 mA·h/g 100 mA·h/g 180 mA·h/g 150 mA·h/g 115 mA·h/g 160 mA·h/g 220 mA·h/g Specific energy 0.518 kW·h/kg 0.400 kW·h/kg 0.630 kW·h/kg 0.495 kW·h/kg 0.414 kW·h/kg 0.576 kW·h/kg 0.920 kW·h/kg 372 mA·h/g 0.0372-0.0744 kW·h/kg 160 mA·h/g 4212 mA·h/g 1624 mA·h/g 0.16-0.32 kW·h/kg 2.106-4.212 kW·h/kg 1.137-1.949 kW·h/kg Lithium rechargeable battery (2) The following equations are in units of moles, making it possible to use the coefficient x. LiCoO2 Li1 x CoO 2 xLi xe xLi xe 6C Lix C6 Overdischarge supersaturates lithium cobalt oxide, leading to the production of lithium oxide Li LiCoO2 Li2O CoO Overcharge up to 5.2 Volts leads to the synthesis of cobalt(IV) oxide LiCoO2 Li CoO2 In a lithium-ion battery the lithium ions are transported to and from the cathode or anode, with the transition metal, cobalt (Co), in LixCoO2 being oxidized from Co3+ to Co4+ during charging, and reduced from Co4+ to Co3+ during discharge. http://en.wikipedia.org/wiki/Lithium-ion_battery Exercises 1 1. What is the internal energy of 1 mole Ar at 0°C ? 2. What is the volume of 1 mole hydrogen gas at 25 °C ? 3. What is the entropy change in 1 mole hydrogen gas at standard conditions when increasing the volume to DV = 1 m3 ? 4. The equilibrium constant for acetic acid in water at 25°C is 4,76. What is Gibbs Free Energy at that temperature ? 5. Calculate the maximum electrical voltage for the DANIELL element when normal pressure and 10 °C ! 6. Calculate the maximum electrical voltage for a galvanic cell with Ni/Ni++//Zn++/Zn when normal pressure and 10 °C ! 7. Explain the difference between a galvanic and an electrolytic cell ! 8. What is the standard hydrogen potential ? Exercises 2 9. What is the standard metal potential ? 10. How can you decide whether an ion will precipitated at a given electrode ? 11. What is the electrode potential for a silver electrode at 10°C when the Ag+ concentration is 1 mol ? 12. How can you calculate the amount of elementary metal to be formed on an electrode ? 13. How can you calculate the maximum energy which can be obtained from a battery 14. Explain the chemical potential ! 16. Explain the lead/acid battery ! 17. Explain the mercury battery ! Web Links • • • http://en.wikipedia.org/wiki/Electrochemistry#Principles http://www.jesuitnola.org/upload/clark/TeachResource.htm http://goldbook.iupac.org/ References • A. Burrows, A. Parsons , G. Price, J. Holman , G. Pilling; Chemistry: Introducing inorganic, organic and physical chemistry ; Oxford University Press 2009 • J. Hoinkins; E. Lindner; Chemie für Ingenieure; Verlag: Wiley-VCH Verlag GmbH & Co. KGaA, 2007 • P.W. Attkins; L. Jobnes; Chemie – einfach alles; Verlag: Wiley-VCH Verlag GmbH & Co. KGaA, 2006 • Römpp‘s Chemie Lexikon • DTV-Atlas zur Chemie