Born-Haber Cycles and Lattice Enthalpies
advertisement

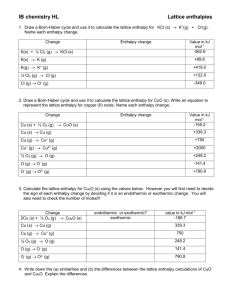
Title: Lesson 6 Born-Haber Cycles and Lattice Enthalpies Learning Objectives: – Understand the term lattice enthalpy – Use Born-Haber cycles to calculate lattice enthalpy – Identify and explain trends in lattice enthalpy Refresh The standard enthalpy change of three combustion reactions is given below in kJ. 2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(l) 2H2(g) + O2(g) → 2H2O(l) C2H4(g) + 3O2(g) → 2CO2(g) + 2H2O(l) ∆Ho = –3120 ∆Ho = –572 ΔHo = –1411 Based on the above information, calculate the standard change in enthalpy, ∆Ho, for the following reaction. C2H6(g) → C2H4(g) + H2(g) Main Menu Bond Enthalpies Look at the covalent bond enthalpies on Table 10 in the Data Booklet. Why do you think there are no ionic bond enthalpies? Main Menu Recap of First Ionisation Energies and Electron Affinities We know that metals loses electrons and non-metals gain electrons. We can use Ionisation Energies and Electron Affinities to work out the enthalpy changes within an ionic compound. The first ionisation energy is the energy needed to remove one mole of electrons from one mole of gaseous atoms: Sodium (on the left of the periodic table) has a relatively low ionisation energy. The first electron affinity is the enthalpy change when one mole of gaseous atoms attracts one mole of electrons.Values can be found in section 7 of the IB data booklet. As the electron is attracted to the positively charged nucleus of the Cl atom, the process is EXOTHERMIC Main Menu Lattice Enthalpies Add the equations for the first ionisation energy and first electron affinity. The process is ENDOTHERMIC overall. This is energetically UNFAVOURABLE (despite the fact it leads to the formation of ions with stable noble gas configurations). Oppositely charged ions come together to form an ionic lattice. The strong attraction between the oppositely charged ions means its very EXOTHERMIC. Lattice Enthalpy Hθlat expresses this enthalpy change in terms of the reverse ENDOTHERMIC process. The lattice enthalpy relates to the ‘formation of gaseous ions from one mole of a solid crystal breaking into gaseous ions’. (As seen above) Main Menu The lattice enthalpy relates to the enthalpy change of ‘formation of gaseous ions from one mole of a solid crystal breaking into gaseous ions’. Think of lattice enthalpy as ‘lattice disassociation enthalpy’... Main Menu Lattice Enthalpy, Hlat This is the equivalent of ‘bond strength’ for ionic compounds It is the enthalpy change when one mole of an ionic compound is converted to gaseous ions. This is an endothermic process, requiring energy to be put in. MX(s) M+(g) + X-(g) Compound Lattice Enthalpy kJ mol-1 LiF 1049 LiBr 820 KF 829 CaF2 2651 Note: Most places define lattice enthalpy the opposite way round, i.e: M+(g) + X-(g) MX(s) The values would be the same magnitude, but with a negative sign to show they are exothermic. It is just a strange quirk of the IB that they do it this way round….I think so that it fits with average bond enthalpies, which are also represent bond breaking Main Menu Born-Haber Cycle Construction of a Using the ‘FAIL’ technique eg for sodium chloride: NB: Hθ f = formation HθIE = ionisation Hθat = atomisation HθEA = electron affinity HθLAT = lattice enthalpy F = formation A = atomisation I = ionisation L = lattice enthalpy Na+ (g) + e- + Cl- (g) Hθ1st IE Na HθEA Cl IONISATION Na (g) + Hθat Na LATTICE H NaCl ENTHALPY θ Cl (g) Hθat Cl ATOMISATION H NaCl Na (s) + FORMATION ½ Cl (g) θ 2 f LAT NaCl (s) Born-Haber Cycle : Applying Hess’s Law There are two routes from elements to ionic compound The Indirect route and the Direct route Clockwise Na+ (g) + e- + Cl- (g) Hθ1st IE Na Na (g) + Hθat Na Na (s) + HθEA Cl Hθ LAT NaCl Cl (g) Anti-clockwise Hθat Cl ½ Cl2 (g) Apply Hess’s Law: = Hθ f NaCl NaCl (s) Clockwise arrows must equal the Anti-clockwise arrows HatNa + HatCl + H 1st IENa + HEACl - HLATNaCl = Hf NaCl Born-Haber Cycle: Calculation If you want to calculate HLATNaCl, it is as follows:HatNa + HatCl + H 1st IENa + HEACl - HLATNaCl = Hf NaCl Rearrange to find the lattice energy: HLATNaCl = [HatNa + HatCl + H 1st IENa + HEACl] - Hf NaCl Values: HatNa = 107 (kJmol-1) HEACl = -349 HatCl = 121 H 1st IENa = 496 Hf NaCl = -411 It’s important to keep all numbers in ( ), whether +ve or –ve, when entering information into your calculator. HLATNaCl = [(107) + (121) + (496) + (-349)] - (-411) HLATNaCl = 786 kJmol-1 So Born-Haber cycles can be used to calculate a measure of ionic bond strength based on experimental data. Born-Haber Cycle Construction of a Using the ‘FAIL’ technique eg for magnesium chloride: NB: Hθ f = formation Hθat = atomisation HθIE = ionisation HθEA = electron affinity HθLAT = lattice enthalpy F = formation A = atomisation I = ionisation L = lattice enthalpy Mg2+ (g) + 2e- + 2Cl- (g) IONISATION 2xH H Mg θ 1st +2nd IE θ EA Cl LATTICE H MgCl ENTHALPY θ Mg (g) + Hθat Mg 2Cl (g) LAT 2xHθat Cl ATOMISATION H MgCl Mg (s) + Cl (g) FORMATION θ 2 f 2 MgCl2 (s) 2 Born-Haber Cycles Lattice enthalpies are difficult to measure directly, so we use Born-Haber cycles These are just a specialised type of Hess cycle To calculate Hlat : Start at the bottom and work round clockwise, adding and subtracting according to the arrows. ionised metal and atomised non-metal Ionisation energy/s (Metal) Electron affinity/s (Non-metal) atomised metal and atomised non-metal Enthalpy of atomisation (metal) ionised metal and ionised non-metal metal and atomised non-metal Enthalpy of atomisation (non-metal) elements in their standard states Enthalpy of formation solid ionic compound Main Menu Hlat = ? Building Born-Haber Cycles Work through the activity here Cut out the equations first and arrange them sensibly on your desk. We will work through LiF together. Hint: Don’t forget to include both ionisations for Mg/Ca and both electron affinities for O Main Menu Main Menu Main Menu Main Menu Solutions Main Menu Main Menu Main Menu