Slide 1
advertisement

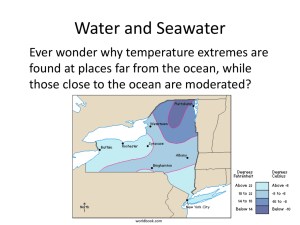
PHYSICAL PROPERTIES OF SEAWATER How Unique is Water? • Water is one of only 3 naturally occurring liquids (mercury and ammonia) • Only substance occurring naturally that exists in all 3 states – solid, liquid, and gas – on Earth’s surface • Extremely large liquid range (0oC - 100oC) • Expands, becomes less dense as a solid The Nature of Pure Water • Water made of 2 hydrogen atoms and 1 oxygen atom • Water is a polar molecule (+ end & – end) • Water’s unusual structure causes them to “stick together” • Water molecules form hydrogen bonds • Hydrogen bonds not very strong, but make water different from any other substance on Earth Figure 3.1 Water has… • Cohesion – sticks to itself • Adhesion – sticks to others • Surface Tension – a measure of how difficult it is to stretch or break the surface of water – No waves without surface tension Water has… • Low Viscosity – little resistance to flow • Good for Earth’s marine organisms – WHY? The Three States of Water • Water is the only substance that naturally occurs as a solid, liquid and gas on Earth • Evaporation absorbs heat • Condensation releases heat Water is Weird • Density – the mass of a certain volume of a substance • Solid water is less dense than liquid water • Water becomes more dense as it cools – Water is most dense @ 4º C – Becomes less dense as it nears 0º C • Good for the planet – WHY? Heat Capacity • The amount of heat required to raise the temperature of 1 gram of a substance 1º C – Water has one of the highest – 1 calorie raises 1 gram of water 1º C – It absorbs large amounts of energy before the temperature changes • Good for earth’s climate – WHY? Changes of State • Latent Heat of Fusion / Melting – – The amount of heat required to melt a substance without change in temperature – 80 calories per gram Changes of State • Latent Heat of Vaporization / Condensation– – The amount of heat required to change a substance from a liquid to a gas without change in temperature – 540 calories per gram Why is Water Important? • Water is the universal solvent • Water can dissolve more than any other natural substance • Water can dissolve many hydrophilic substances – Ionic compounds – Other polar compounds • Form “spheres of hydration” • http://programs.northlandcollege.edu/biology /Biology1111/animations/dissolve.html Figure 3.5 Seawater has dissolved solids… • Source – – Chemical weathering of crustal rocks – Hydrothermal vents – From volcanic eruptions Water … • Density of pure water is 1 g/mL @ 4º C • Density of seawater is 1.0278g/mL @ 4ºC • Density is determined by temperature and salinity – Seawater gets denser as it gets saltier, colder or both • Because temperature varies more than salinity, density is controlled by temperature Density… • • • • Changes with depth Densest water sinks Ocean becomes layered, stratified Seen in profiles of salinity, temperature, and density • The greater the difference in density between surface and deep water, the more stable the water column and the harder it is to mix vertically Figure 3.31 Pressure… • Water is noncompressible – does not change volume with increasing pressure • So is seawater • Pressure increases with increasing depth – Has small effect on volume – 1 atm (14.7 lbs/in2) for every 10 m (33 ft) – Pressure in deepest trench ~1100 atm – As pressure increases, gases are compressed Figure 3.13 Buoyancy • The ability of an object to float by displacing a volume of water equal to its own weight Water… • Transmits energy – Heat – Light – Sound • Refraction – the bending of light and sound waves due to density differences that affect the speed of energy transmission – increases with increasing salt, decreases with increasing temperature Water… • Transmits heat energy by – Conduction – molecule to molecule – Convection – moving fluids & density driven – Radiation – direct from source (sun) Water is … • Transparent – transmits light energy readily – Important for photosynthesis – Oceans are blue because blue light penetrates the deepest – Coastal waters sometimes green because blue absorbed Water… • Transmits sound faster & farther than in air – 1500 m/s in seawater (& 60 times farther) – 334 m/s in air • At 1000 m combination of salinity, temp & pressure creates a zone of minimum velocity for sound – the Sofar Layer (sound fixing and ranging layer) – Sound waves produced here do not escape & travel long distances Sofar Channel