blog 4-1 Thermochemistry
advertisement

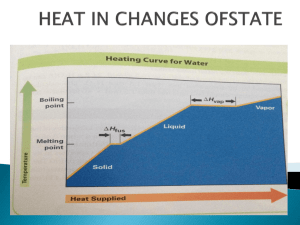
Chapter 17 – Thermochemistry 4-1 -is the study of energy ( heat) relationships in chemical and physical reactions 1 • Thermochemistry is concerned with the flow of heat from the system to it’s surroundings, and vice-versa. • almost nothing happens in chemistry unless there is an energy advantage; all reactions are an attempt to arrive at a lower energy within the system. a) 1st law of Thermodynamics: Law of conservation of energy. • Energy is never created nor destroyed , it only changes form. This law states that energy can change form but is never really lost in any closed system. b) 2nd law of Thermodynamics: • Heat Energy always travels spontaneously from a warmer body (body with higher temperature) to a colder body. -dropping a hot rock in water -frostbite • temperature is simply an indirect measure of the average kinetic energy of the particles in a sample of matter. i) Celsius scale sets the zero value at the freezing point of water at 101.325 kPa and 100 at the boiling point of water at the same pressure. ii) Kelvin scale sets the zero value at absolute zero. Remember: 0K = -273.15 °C K = t + 273.15 °C Energy and Heat • Energy - capacity for doing work or supplying heat – if within the chemical substancescalled chemical potential energy 5 • Heat - represented by “q”, is energy that transfers from one object to another, because of a temperature difference between them. 6 • Essentially all chemical reactions, and changes in physical state, involve either: – release of heat, (exothermic) or – absorption of heat (endothermic) • heat flowing into a system from its surroundings: – q has a positive value – called endothermic – Absorbs in the form of heat • system gains heat as the surroundings cool down • heat flowing out of a system into it’s surroundings: – q has a negative value – called exothermic – Releases in the form of heat • system loses heat as the surroundings heat up • Every reaction has an energy change associated with it • Exothermic reactions release energy, usually in the form of heat. • Endothermic reactions absorb energy • Energy is stored in bonds between atoms 10 Heat Capacity and Specific Heat • A calorie is defined as the quantity of heat needed to raise the temperature of 1 g of pure water 1 oC. 11 • The calorie is related to the joule, the SI unit of heat and energy – 4.184 J = 1 cal • Heat Capacity - the amount of heat needed to increase the temperature of an object exactly 1 oC 12 • Specific Heat Capacity - the amount of heat it takes to raise the temperature of 1 gram of the substance by 1 oC often called simply “Specific Heat” • mass; measured in g or kg. The greater the mass, the more heat required to change the temperature. 13 • For water, C = 4.18 J/(g oC), • Thus, for water: – it takes a long time to heat up, and – it takes a long time to cool off! • Water is used as a coolant! 14 • • • • • • • To calculate, use the formula: q = m C T heat abbreviated as “q” m = mass T = change in temperature (Tf - Ti) C = Specific Heat Units are either J/(g oC) or kJ/(kg oC) – J/(g K) or kJ/(kg K) 15 Table of Specific Heats Ex: Determine the heat required to raise the temperature of 100.g of water from 298.0 K to 373.0 K . Q = m c ΔT Q =100.g (4.184 J/g K)( 373.0 K –298.0 K) Q = 418.4 J/K (75 K) Q = 31350 J Q = 31.4 kJ 17 Molar heat capacity • Cp0 • of a substance is the amount of energy required to raise the temperature of one mole of a substance by one Celsius degree. • Molar heat capacity • = (specific heat) (molar mass) • Calculate the molar heat capacity of water, given that the specific heat water is 4.184 J/g°C. Cp0 = (specific heat)(molar mass) = (4.184 J/g°C)(18.02g/mol) = 75.40 J/mol °C