here
advertisement

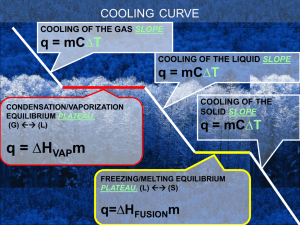
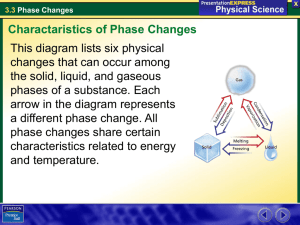
Name: Date: Block: Phase Changes* 1. Label each state of matter involved in a phase change. 2. Label the phase changes with arrows and a + or -. Answer the questions below. 1. Which container(s) represents vaporization? __________________ 2. In which container(s) is the water boiling? 3. __________________ In which container(s) is the water evaporating? _________________ 4. How are these two types of vaporization similar? (Look at the definition of vaporization.) 5. How is boiling different from evaporation? 6. Did the wax gain energy when it froze or when it melted? Pick one and explain. 7. Did the wax lose energy when it froze or when it melted? Pick one and explain. 8. Why are candles frozen at room temperature? (Think of what state of matter candles are at room temperature) 9. Name two substances that are frozen at room temperature. Remember frozen doesn’t mean cold! 1.___________________________ 2____________________________ 10. The candle’s melting point is higher, lower or the same as its freezing point. Circle one. 12. Name the phase change the occurs when the water vapor touches the mirror.__________________________________ 13. The phase change named in number 12 causes the water to go from a ______________ to a ________________ (states of matter). The energy of the water molecules increase/decreases. (Circle one) 14. What is an example of condensation you see in everyday life? 15. Label the graph with the terms in the box. Solid Liquid Gas Melting/Freezing Point Boiling/Condensation Point Complete the table below.