Covalent Bonding
advertisement

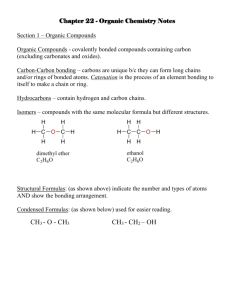
What is a covalent bond? A covalent bond forms between two atoms that are sharing one or more pair of electrons. Usually occurs when the electronegativity difference between the two elements is small (<1.7) Covalent bonds form between two nonmetals. The bond results from an overlapping of orbitals. A covalent bond is a weaker bond than an ionic bond. Properties of compounds resulting from a covalent bond include: low melting point, gases or liquids at room temp (some are soft solids), do not conduct electricity, many are insoluble in water. Naming covalently bonded compounds. The first element is named according to the element name. An –ide ending is added to the name of the second element. Numerical prefixes are used to indicate the number of each type of atom present. Numerical Prefixes Prefix Number *Mono (only used with the second element) 1 Di 2 Tri 3 Tetra 4 Pent 5 Hex 6 Hept 7 Oct 8 Non 9 Dec 10 Name the following compounds CO2 CO N2O NO NO2 N2O3 N2O4 N2O5 PCl5 PCl3 SF6 Write the formulas for the following compounds: Sulfur trioxide Dioxygen difluoride Tetraphosphorus decaoxide Diboron trioxide Arsenic pentafluoride Silicon dioxide Monohydrogen dioxide Nitrogen trihydride Types of Covalent Bonds A single covalent bond occurs when one pair of electrons (2 electrons) are shared between two atoms. If the electrons are shared equally, it is called a nonpolar covalent bond. (This type of bond only occurs if the electrons are shared between identical atoms) If the electrons are shared unequally, it is called a polar covalent bond. Polar and Nonpolar Molecules Molecules can also be polar and nonpolar. Molecules are nonpolar if: 1) the bonds are nonpolar 2) the polar bonds are arranged symmetrically Molecules are polar if the polar bonds are arranged asymmetrically. Lewis Dot Diagrams Cl2 Linear Nonpolar bonds Nonpolar molecule Chlorine Lewis Dot Diagrams H2O Bent Polar bonds Polar molecule Dihydrogen monoxide Lewis Dot Diagrams NH3 Trigonal pyramidal Polar bonds Polar molecule Nitrogen trihydride Lewis Dot Diagrams CH4 Tetrahedral Polar bonds Nonpolar molecule Carbon tetrahydride In covalent compounds, atoms become stable by ___ their valence electrons. 5% 95% transferring 2. sharing 1. A ___ covalent bond is the result of an equal share of electrons by both atoms. 11% 89% polar 2. nonpolar 1. A cation has a ___ charge. 100% 0% positive 2. negative 1. When an atom ___ electrons, it becomes positively charged. 5% 95% gains 2. loses 1. In the formula CO2, the number 2 is called a ___. 0% 0% 0% 100% superscript 2. oxidation number 3. charge 4. subscript 1. What is the correct name for PCl3? 0% 0% 100% 0% Monophosphorus trichloride 2. Phosphorus chloride 3. Phosphorus trichloride 4. Traphosphorus chloride 1. What is the correct name for Li2O? 11% 37% 26% 26% Lithium oxide 2. Dilithium oxide 3. Lithium (II) oxide 4. Lithium dioxide 1. Which is not a diatomic molecule? 11% 22% 11% 56% Fluorine 2. Nitrogen 3. Bromine 4. Boron 1. Which element is a nonmetal? 11% 0% 89% 0% Boron 2. Lithium 3. Carbon 4. Magnesium 1. Which element has 7 valence electrons? 0% 0% 100% 0% Boron 2. Nitrogen 3. Fluorine 4. Manganese 1. Noble gases are stable because they have __ valence electrons. 0% 0% 0% 100% 2 2. 4 3. 6 4. 8 1. The noble gases are in which block of the periodic table? 6% 61% 22% 11% s 2. p 3. d 4. f 1. Electrons have what charge? 84% 16% 0% -1 2. +1 3. 0 1. How many valence electrons are in an atom of oxygen? 0% 0% 100% 0% 2 2. 4 3. 6 4. 8 1. How many shared pairs are present? 0% 100% 0% 0% 1 2. 2 3. 3 4. 4 1. How many electrons are not shared? 5% 26% 0% 68% 1 2. 2 3. 3 4. 4 1. How many single bonds are present? 5% 63% 0% 32% 1 2. 2 3. 3 4. 4 1. Are the bonds polar or nonpolar? 74% 26% Polar 2. Nonpolar 1. What is the shape of the molecule? 21% 63% 0% 16% Linear 2. Bent 3. Trigonal pyramidal 4. Tetrahedral 1. Is the molecule polar or nonpolar? 63% 37% Polar 2. Nonpolar 1. 10 ml of the substance has a mass of 9 g. What is its density? 79% 5% 16% 0% 0.9 g/ml 2. 1.0 g/ml 3. 1.1 g/ml 4. 19 g/ml 1. What is the percent oxygen in the substance? 11% 61% 22% 6% 11% 2. 33% 3. 67% 4. 89% 1. Double and Triple Bonds A double covalent bond occurs when two atoms share two pairs (4 electrons) of electrons. A triple covalent bond occurs when two atoms share three pairs (6 electrons) of electrons. Examples of compounds consisting of double bonds. CO2 O2 C2H4 Examples of compounds consisting of triple bonds. N2 HCN C2H2