Ideal Gas Introduction and Practical
advertisement

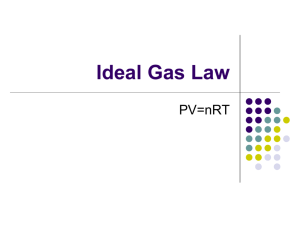
Title: Lesson 10 Ideal Gas Equation Learning Objectives: – Understand the ideal gas equation – Complete a circus of short experiments to explore the ideal gas equation – Perform calculations using the ideal gas equation Molar Volume of a Perfect Gas We learnt about the molar volume of gases last lesson….how can they be the same? The distance between particles is much bigger than the size of the particles….so particle size makes very little difference: The blue particle is twice the size of the red particle, but the blue particles are not taking up twice the amount of space. 10 units 10 units In reality, the relative distance between the molecules is much much greater than this. Main Menu The Ideal Gas Equation The volume a gas takes up is determined by: Pressure Temperature Moles of gas The value of the constant is directly proportional to the fixed mass of gas, or the number of moles, n This combines to form the ideal gas equation PV = nRT Where: P = pressure in Pa V = volume in m3 n = moles of gas R = gas constant, 8.31 J K-1 mol-1, this appears in many places in chem T = temperature in K Calculating the Mr of gases 4 of 29 © Boardworks Ltd 2009 Using the ideal gas equation 5 of 29 © Boardworks Ltd 2009 Ideal Gas Assumptions Particles occupy no volume Particles have zero intermolecular forces These are not always valid, particularly at: Low temperature High pressure Main Menu Study the equation to make some predictions: PV = nRT How would does temperature change if you decrease pressure at fixed volume? If you decrease pressure, temperature will decrease. How would volume change if you heat something at fixed pressure? If you increase temperature, volume will increase. How would pressure change if you decrease the volume at fixed temperature? If you decrease volume, pressure will increase. Main Menu Exploring Ideal Gases The best way to explore the behaviour of gases is to have a little play. Complete these four short experiments Main Menu