File
advertisement
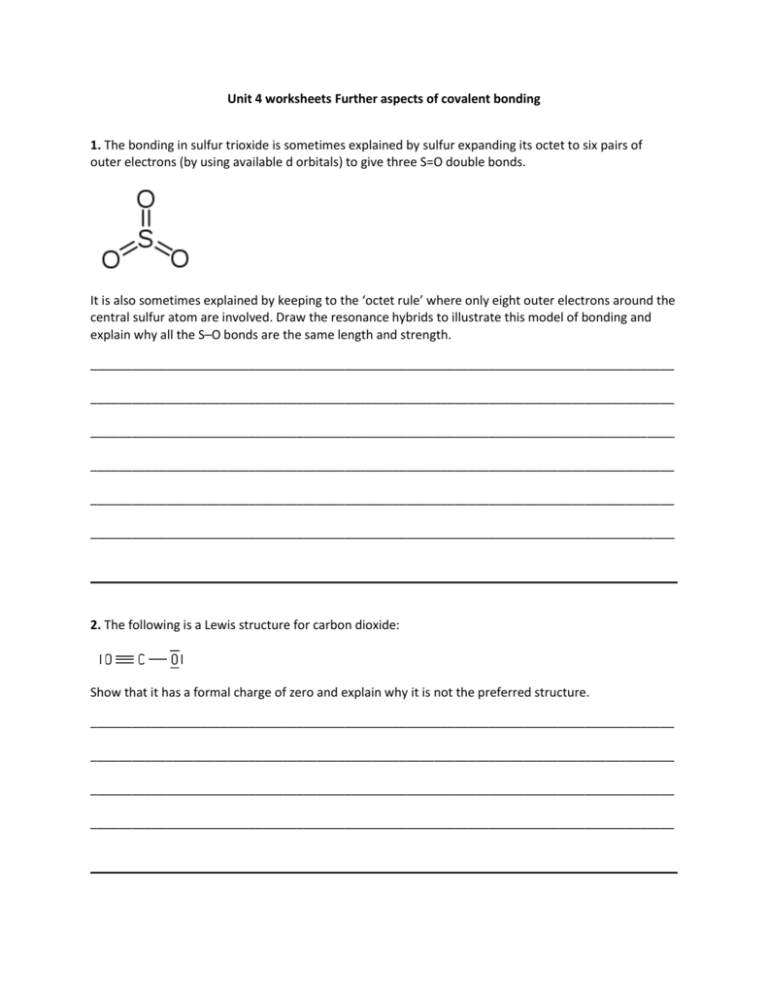
Unit 4 worksheets Further aspects of covalent bonding 1. The bonding in sulfur trioxide is sometimes explained by sulfur expanding its octet to six pairs of outer electrons (by using available d orbitals) to give three S=O double bonds. It is also sometimes explained by keeping to the ‘octet rule’ where only eight outer electrons around the central sulfur atom are involved. Draw the resonance hybrids to illustrate this model of bonding and explain why all the S–O bonds are the same length and strength. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 2. The following is a Lewis structure for carbon dioxide: Show that it has a formal charge of zero and explain why it is not the preferred structure. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 3. Explain why the two C–O bond lengths in propanoic acid, C2H5COOH, are different and yet the two C–O bond lengths in the propanoate anion, C2H5COO–, are the same length. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 4. Explain why p orbitals on one atom can form two pi bonds and one sigma bond when they combine with the p orbitals on another atom. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 5. Ultraviolet light can break the oxygen to oxygen bond in both oxygen, O2, and ozone, O3, molecules. The O=O bond energy is 498 kJ mol-1. Calculate the wavelength of light required to break this bond. Suggest why the light required to break the O–O bond in oxygen is of a higher frequency than the light required to break the O–O bond in ozone. _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ 6. When there is a large amount of delocalised electrons in a molecule the molecule tends to be coloured. The two structures below are for the indicator phenolphthalein in (a) acid solution and (b) in alkali solution. Explain why phenolphthalein becomes coloured in an alkaline solution (a) in acid solution (b) in alkali solution _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ Answers 1. The S–O bonds are the same length and strength as the real structure lies between these three extreme resonance structures with identical S–O bonds with an average of one and a third bonds each (a bond order of 4/3). 2. C = 4 – 0 – (½ x 8) = 0, O = 6 – 2 – (½ x 6) = +1 (O with triple bond) O = 6 – 6 – (½ x 2) = – 1(O with single bond) so overall charge = 0 However in the preferred structure with two double bonds all the atoms within the molecule each have a charge of zero. 3. In propanoic acid, C2H5COOH, there is a C–O single bond and a C=O double bond. The C–O single bond is longer than the C=O double bond. In the propanoate ion delocalisation occurs so that the extra electron is shared between the two C–O bonds giving two identical bonds with an average 1.5 bond which has a bond length between a C–O single bond and a C=O double bond. 4. Two of the p orbitals combine ‘sideways’ to form pi bonds whereas the other p orbital combines ‘head on’ forming a sigma bond. 5. For just one double bond this equates to 498 divided by Avogadro’s constant = 8.27 x 10-19 J. The wavelength of light that corresponds to this enthalpy value (E) is calculated by combining the expressions E = hν and c = λν to give λ = λ= 6.63 x 10-34 (Js) x 3.00 x 108 (ms-1) / 8.27 x 10-19 (J) = 241 nm The double O=O bond in oxygen is stronger than the 1.5 O-O bond in ozone (the average of the two resonance hybrid structures) so more energy (higher frequency) is required to break the O-O bond in oxygen. 6. The hybridization of the central carbon atom changes from sp3 to sp2. This provides a single electron in a p orbital so that the electrons can delocalise throughout the whole anion via conjugated π bonds whereas in the undissociated molecule the delocalisation is limited mainly just to the separate aromatic rings.







