RESTLESS LEGS SYNDROME (RLS) Symptom Derived Treatment
advertisement

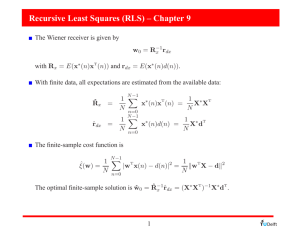
RESTLESS LEGS SYNDROME (RLS) Symptom Derived Treatment Philip M. Becker, M.D. Medical Director, Sleep Medicine Institute, Presbyterian Hospital Clinical Professor, Dept. of Psychiatry, UT Southwestern Medical Center Dallas, Texas Patients describe RLS in a multitude of colorful ways A compelling sensation occurring at rest that creates URGE to move. Described, often with difficulty or in dramatic terms, as: "creepy, crawly, jumpy" "heebie jeebies" "jerky or jittery" "ants or worms crawling inside" "agitation, anxious" but just in the limbs (unless very severe) "hurts deep inside but it isn't really pain" IRLSSG Diagnostic Criteria Four Core Symptoms are required “U R G E” U = Urge to move the limb(s) R = Rest worsens the sensation G = Get up & Go is Good (temporary relief with movement) E = Evening / night worsening Current Hypothesis on Etiology • Dopaminergic Mechanism in RLS – Success of dopaminergic therapy supposes a mechanism of action. – Four studies have identified small reductions (10-20%) in striatal dopamine activity. • Iron Dysregulation in the CNS – Conner et al. found reduced iron transporter in nigral neurons of RLS patients compared to control brains. – Serum ferritin less than 30 increases the likelihood of improvement with iron. – CSF ferritin reductions correlate with RLS severity. – Iron stores in the substantia nigra of RLS patients are lower on fMRI. Epidemiology of RLS P r e v a l e n c e P r e 1 9 7 0 2 5 % ( 3 s t u d i e s ) S i n c e 1 9 9 49 1 5 % ( 5 s t u d i e s ) P r o g r e s s i o n O n s e t : 4 3 % d e v e l o p R L S b e f o r e a g e 2 0 I n c r e a s e i n c i d e n c e u p t o a g e 6 9 ( & o l d e r ) I n c h i l d r e n w h o h a v e p a r e n t s w i t h R L S , P L M D f o u n d m o r e c o m m o n l y t h a n R L S . P L M S n o t e d i n 8 0 % o f R L S p a t i e n t s o n N P S G # 1 ( a n d + 7 . 6 % o n N P S G # 2 ) . Primary Restless Legs Syndrome presents generally before age 30. Etiology of primary RLS is unknown. About 50% of primary patients have a positive family history for RLS. Genetics: • Trenkwalder: probable autosomal dominant inheritance with high penetrance and variable expressivity. • Desaulters: Chromosome 12q in French-Canadians There must be no medical or substances to account for the symptoms. Secondary RLS arises more commonly after age 30. It arises in or from other conditions such as: Uremia 23 - 30% report RLS Pregnancy 17% report RLS Neuro Disorders: spinocerebellar ataxia, CMT 2, Small fiber neuropathy, DM, lumbar radiculopathy Metabolic Deficiency: IRON, folate, B12, thyroid, others Rheumatoid Arthritis Treatment focuses upon correction of the medical disorder. Management Strategies for RLS B e h a v i o r a l I n t e r v e n t i o n s S e c o n d a r y D i s o r d e r s r e q u i r e a c t i v e c o r r e c t i o n a n d t r e a t m e n t . P h a r m a c o l o g i c T h e r a p i e s Target Symptoms for RLS Therapy l e v e l t s e w o l e h t o t e c u d e r a i s e h t s e s y D . s r u o h 4 2 e h t t u o h g u o r h t e l b i s s o p p e e l s , t e s n o p e e l s e z i l a m r o N : p e e l S . s s e n t r e l a e m i t y a d d n a , e c n a n e t n i a m n a o t e c u d e R : s t n e m e v o M g e L c i d o i r e P d n a t n e i t a p r o f l e v e l c i t a m o t p m y s a . r e n t r a p d e b t n a c i f i n g i s e r o f e b t n e m t a e r t f o g n i m i T . s m o t p m y s f o t e s n o . d e z i m i n i m e b d l u o h s s t c e f f E e d i S Behavior Modification for RLS A v o i d c a f f e i n e , c h o c o l a t e , a n d M S G . A e r o b i c e x e r c i s e , b u t b e f o r e 7 : 0 0 p . m . L i m i t u s e o f c e n t r a l l y a c t i v e s t i m u l a n t a g e n t s : D e c o n g e s t a n t s , a n t i h i s t a m i n e s , n i c o t i n e , a p p e t i t e s u p p r e s s a n t s , e t c . A v o i d S S R I a n t i d e p r e s s a n t s , u n l e s s o n l y e f f e c t i v e t r e a t m e n t ( P o s s i b l y b e t t e r : b u p r o p r i o n o r n e f a z o d o n e ) . C o u n t e r s t i m u l i : s o c k s , s t r e t c h i n g , h o t b a t h o r s h o w e r , i c e p a c k , o t h e r s . Pharmacologic Treatment Experience for RLS • Opiates • Benzodiazepines 300 + years 25 + years • Clonazepam & others • Levodopa • Dopamine Agonists 15 + years 5-10 years • Ropinirole, pramipexole, others • Anti-convulsants 5 + years • Gabapentin • Others (iron, mg, calcium, B vitamins, magnets, etc.) Complex RLS Case • 54 y/o W/M man with 20+ year hx of RLS. • Renal disease with creatinine of 2.1, stable. • Hospitalized for major depression 4 yrs ago; currently taking fluoxetine at 40 mg/d. • “My legs are driving me nuts. Inside they crawl and hurt. I just have to move them.” • “I can’t live this way any longer. I would be better off dead.” • Taking carbidopa/levodopa ER 50/200 qid for 8 months. Relief of 1-2 hours, otherwise 24-hour RLS. Treatment Planning for WM Did anything help you? “I slept with Percodan (taken for 3 days after dental work). Sinemet CR “helped for 2 months and then it won’t last. I took more.” Do you have a plan to take your life? “No, I love my kids. It wouldn’t be fair to them.” Recheck creatinine, hemoglobin and hematocrit, B12, folate, and Ferritin. No changes were found. WM has Augmentation to Levodopa • Augmentation: shift of RLS symptoms by two or more hours to an earlier onset compared to the patient’s baseline. • Levodopa produces augmentation in 30-85% of RLS patients (Ave onset: 1-3 months). • Dopamine agonists (DA) estimated to produce augmentation in 15-35% of patients (Onset >6 mos). • Management Options: • For levodopa, taper off then switch to DA. • If DA, consider earlier dosing. Observe for further augmentation. • In the severe patient, shift to high potency opiate. • Consider gabapentin, clonazepam or other therapy. Initial Treatment Plan for WM Taper carbidopa/levodopa ER 50/200 by one tablet every 2 days, beginning at h.s., to prevent augmentation. To reduce augmentation, substitute ropinirole 0.25 mg for each of the c/l dopa ER tablets every 2 days. Oxycodone 5 mg p.o. h.s. for acute symptom relief and to improve sleep. Emphasized complexity, need for daily contact and possible need to hospitalize. Comparison of Dosing of Dopaminergic Agents for RLS Agent Brand Name Initial Dose (p.o.) in mg Dosage Timing (h.s. RLS) Dosing Adjustment Recom. Maximum Dose (mg) levodopa Sinemet & others 25/100 1/2 - 1 @ h.s. 1 q 3d 300* (total) C/Ldopa ER Sinemet CR 25/100 1 1 q 3d earlier pm 300* (total) pergolide PERMAX 0.05 pramipexole MIRAPEX ropinirole REQUIP 2hr b4 h.s. 1 q 2-3d 1-2 0.125 1 supper &/or h.s. 1 2hr b4 hs 1 q 2-3 d 1.5 - 3 0.25 1 1 q 2-3 d 4-8 1-2hr b4 h.s. *Levodopa maximum of 300 mg recommended to lessen augmentation. Up to 1200mg/ d has been used. WM Treatment Course Day 1-10 Day 1: “I slept 5 hours.” Day 6: “The legs are 30% better, but I’m only sleeping 4 hours or so.” Response: INCREASE ROPINIROLE DOSES #3&4 to 0.50 mg. Day 10: “My legs are OK until about 11:00 and then they act up. The medicine makes me really sick (nauseated).” Response: Nausea should get better. (I wish domperidone was available in U.S.) INCREASE ROPINIROLE at noon to 0.5 mg. Limitations to Therapeutic Benefit Side effects Dopamine agonists: nausea, edema, myalgia, sleepiness, insomnia, augmentation Opioids: sleepiness (? SDB), insomnia, constipation, nausea, “fear of addiction” Loss of efficacy Consider anemia/iron deficiency. Need for dose adjustment Is it augmentation? WM Treatment Course Day 11-14 Day 11: “I’m sick all day. I didn’t sleep.” Response: Discontinue a.m. ropinirole. Day 12: “You got to do something.” Response: Reduce ropinirole to 0.25 mg noon & 6:00 pm, continue 0.5 mg at 10:00 pm. Day 14: “I’m not as sick but my legs are worse. Is this ever going to get better?” Response: Add oxycodone 5 mg at 6:00 p.m. (and h.s.). Reduce ropinirole to 0.25 mg TID. WM Treatment Course Day 21-24 Day 21: “I’m 50 % better. I even slept 6 hours one night.” RL starts at about noon. Response: Increase ropinirole to 0.5 mg with lunch. Day 24 “That stuff makes me sick. I decreased it (back to 0.25 mg at noon). My legs were getting puffy.” Response: Checked chemistries and renal function. No acute changes. WM Treatment Course Day 26-35 Day 26: “Doc, I think the dope works the best. The fluid was making my legs hurt.” Response: Stopped ropinirole Changed oxycodone to METHADONE 5 mg at 6:00 p.m. and 10:00 p.m. Day 35: “Doc, it’s amazing. I don’t feel my legs jumping and I slept for five nights straight.” Edema resolved. Efficacy of Different Opioids in Treatment of RLS in 18 Severe Patients I R L S S R a t i n g 3 5 S c o r i n g 3 0V . S e v . = 3 1 4 0 e v e r e = 2 1 3 0 2 5S 1 8 . 4 3 M o d . = 1 1 2 0 2 0 M i l d = 1 1 0 1 4 . 3 1 1 5 1 1 . 1 7 1 0 2 6 . 6 7 . 7 5 8 . 58 5 0 l e v o r p h a n o l p r a m i p e x o l e h y d r o m o r p h o n e h y d r o c o d o n ep r o p o x y p h e n eB a s e WM Treatment Course Day 35 + • Depression remained moderate with low energy & motivation; moderate sadness. • WM’s personal doctor agreed to my request to discontinue Prozac. • Wellbutrin SR 150 mg was started and increased to a.m. and noon one week later. • Four weeks later, clonazepam 0.5 mg h.s. was added due to “feeling a bit nervous.” • At eight weeks, he slept >6 hours nightly and said “I feel pretty good.” He has remained so for 3+ years on methadone, buproprion XL and clonazepam. Treatment Considerations for Restless Legs Syndrome • Treat symptoms identified as problems by patient. • Dopamine agonists are the first line, presuming a ferritin > 30. Target therapy before RL onset. • C/L dopa causes augmentation. Best used prn. [Arbitrary limit:300 mg/week ( 3 tabs of 25/100)] • Opiates restore severe patients. High potency agents preferred (methadone, levorphanol, hydromorphone) but caution for EDS and SDB. • Gabapentin is variably effective. Best with “pain”. • Depression and anxiety are common and require treatment when present. Buproprion preferred.