1.14 Naming multivalent compounds HO
advertisement

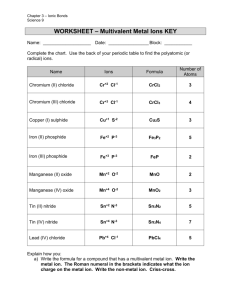
SCH 4C Naming Multi-Valent Ionic Compounds Last time, we learned about simple ionic compounds. Complete the following table. Ion symbol of non-metallic element Name Combining Elements Ion symbol of metallic element Ionic Formula Calcium and Phosphorous Ca2+ P3- Calcium Phosphide Ca3P2 Magnesium and Oxygen Beryllium and Sulfur Sodium and Iodine Lithium and Nitrogen Barium and Chlorine Multivalent metals can lose different numbers of electrons, having more than one valence. Example: Fe3+ and Fe2+ Charge Number (Valence) 1 To avoid confusion when naming the compounds they form, we identify the metal valence using a roman numeral in brackets: 2 Steps to Naming Multivalent compounds: 4 Step 1: ___________________________________________ 5 __________________________________________________ Roman Numeral 3 6 7 Step 2: ___________________________________________ __________________________________________________ Step 3: ____________________________________________________________________________ ___________________________________________________________________________________ Step 4: ____________________________________________________________________________ Try the formula for titanium (IV) fluoride Manganese (III) Sulfide Ions: Ions: Charge: Charge: Balanced: Balanced: Formula: Formula: Now Try these: a) Iron (II) oxide b) Iron (III) oxide c) Copper (II) nitride d) Lead (IV) sulfide Steps for Writing formulas for Multivalent compounds. Step 1: ____________________________________________________________________________ ___________________________________________________________________________________ Step 2: ____________________________________________________________________________ ___________________________________________________________________________________ Step 3: ____________________________________________________________________________ ___________________________________________________________________________________ Step 4: ____________________________________________________________________________ ___________________________________________________________________________________ Step 5: ____________________________________________________________________________ ___________________________________________________________________________________ Step 6: ____________________________________________________________________________ ___________________________________________________________________________________ Try to write the name for FeI2: Is there a multivalent metal? What are its different ion forms? What is the ratio of ions What is the charge on the negative ion Balance the positive and negative charges Write the name using a roman numeral to say which ion form of Iron is present Try another, PbF4: Is there a multivalent metal? What are its different ion forms? What is the ratio of ions What is the charge on the negative ion Balance the positive and negative charges Write the name using a roman numeral to say which ion form of Iron is present Now Try with the Following Formulas: CuCl2 CuSO4 Cu2O CuO 1. FeO 21. Calcium hydroxide 2. SnS2 22. Chromium (III) chloride 3. PbSO4 23. Chromium (II) carbonate 4. Cr 2 S 3 24. Silver sulphate 5. Cu(NO3)2 25. Ammonium fluoride 6. Fe 2 (SO 4 ) 3 26. Iron (III) dichromate 7. SnF2 27. Lead (II) sulphide 8. HgSO4 28. Copper (II) permanganate 9. Cu 3 (PO 4 ) 2 29. Chromium (III) sulphate 10. Mn(MnO4)2 30. Copper (II) fluoride 11. Fe(OH)2 31. Chromium (III) hydrogen carbonate 12. Pb(CrO4)2 32. Iron (III) phosphate 13. CuCl 33. Sodium sulphide 14. MnO2 34. Lead (IV) chloride 15. SnSO4 35. Mercury (I) nitrate 16. Fe(ClO3)2 36. Chromium (II) oxide 17. PbBr2 37. 18. Cu(HS)2 38. Calcium chromate 19. Mn(CO3)2 39. Barium phosphate 20. Pb(NO2)4 40. Tin (IV) sulphate Magnesium nitrate