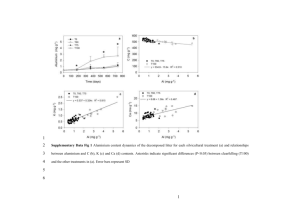
Specific Heat Capacity
advertisement

Image source: http://www.green earthproject.com/ Forms2.html POTENTIAL ENERGY Gravitational Potential Energy Depends on height Elastic Potential Energy Associated with objects that can be stretched or compressed CHEMICAL ENERGY energy required to bond atoms together NUCLEAR ENERGY Image source: http://typesofalternativeenergy.com/ released in the form of light and heat energy as the nucleus splits Most concentrated form of energy > 50,000 DEGREES CELSIUS KINETIC ENERGY MECHANICAL ENERGY energy associated with motion Movement of turbines Image source: http://www.entergy.apogee.net/ Image source: http://technicalstudies.youngester.com Moving electric charges Internal motion of atoms Heat energy causes changes in the temperature and phase (solid, liquid, gas) of any form of matter ENERGY TRANSFORMATIONS http://www.youtube.com/watch?v=RD_54Cq_UMM Changes in Energy http://172.25.31.139/index.asp?ResourceString=MSMC/Physics/ Media Server Online: Potential and Kinetic Energy SPECIFIC HEAT CAPACITY Or the amount of energy needed to heat substances up Specific Heat Capacity can be thought of as a measure of how much heat energy is needed to warm the substance up. You will possibly have noticed that it is easier to warm up a saucepan full of oil than it is to warm up one full of water. http://www.cookwaremanufacturer.com/photo/418fa6490f24202f2cc5b5feee0fdde3/Aluminum-Saucepan.jpg Heat vs. Temperature Heat is the total energy of molecular motion in a substance while temperature is a measure of the average energy of molecular motion in a substance. Heat energy depends on the speed of the particles, the number of particles (the size or mass). Temperature does not depend on the size or type of object. For example, the temperature of a small cup of water might be the same as the temperature of a large tub of water, but the tub of water has more heat because it has more water and thus more total thermal energy. Heat travels by convection, conduction, and radiation. Going from an area of high temperature to an area of low temperature http://www.youtube.com/watch?v=rU-sPzshVnM Specific Heat Capacity (C) of a substance is the amount of heat required to raise the temperature of 1g of the substance by 1oC (or by 1 K). The units of specific heat capacity are J oC-1 g-1 or J K-1 g-1. Sometimes the mass is expressed in kg so the units could also be J oC-1 g-1 or J K-1 kg-1 The next table shows how much energy it takes to heat up some different substances. The small values show that not a lot of energy is needed to produce a temperature change, whereas the large values indicate a lot more energy is needed. Approximate values in J / kg °K of the Specific Heat Capacities of some substances are: Air Aluminum Asbestos Brass Brick Concrete Cork Glass Gold Ice Iron 1000 900 840 400 750 3300 2000 600 130 2100 500 Lead Mercury Nylon Paraffin Platinum Polythene Polystyrene Rubber Silver Steel Water 125 14 1700 2100 135 2200 1300 1600 235 450 4200 The equation: The amount of heat energy (q) gained or lost by a substance = mass of substance (m) X specific heat capacity (C) X change in temperature (ΔT) q = m x C x ΔT AN EXAMPLE OF A CALCULATION USING THE SPECIFIC HEAT CAPACITY EQUATION: How much energy would be needed to heat 450 grams of copper metal from a temperature of 25.0ºC to a temperature of 75.0ºC? (The specific heat of copper at 25.0ºC is 0.385 J/g ºC.) Explanation: The change in temperature (ΔT) is: 75ºC - 25ºC = 50ºC Given mass, two temperatures, and a specific heat capacity, you have enough values to plug into the specific heat equation q = m x C x ΔT . and plugging in your values you get q = (450 g) x (0.385 J/g ºC) x (50.0ºC) = 8700 J SOME GOOD WEBSITES http://www.s-cool.co.uk/gcse/physics/energytransfers/types-of-energy-transfers.html#typesof-energy