AP Biology Functional Groups of Carbon
advertisement

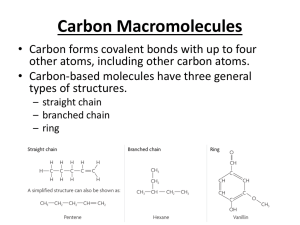
The Major Functional Groups 1. 2. 3. 4. 5. 6. 7. 8. Hydroxyls of alcohols Carbonyls of aldehydes and ketones Carboxyls of carboxylic acids Aminos of amines Sulfhydryls of thiols Phosphates of organic phosphates Methyl Methylene 1. The Hydroxyl The hydroxyl group is an –O-H attached to a hydrocarbon. Called alcohols Usually have “alcohol” or “ol” at the end of the name. Example, the 2-carbon alcohol ◦ Called ethyl alcohol or ethanol Alcohols are the least oxidized (most reduced) of all the oxygen-containing functional groups. Examples of Alcohols • Isopropyl alcohol (rubbing alcohol) – Note –OH is on middle carbon – Soluble in water. • Cholesterol – Note –OH on lower left – Insoluble in water. • Glycerol – Molecule with 3 –OH’s – Soluble in water Properties of Alcohols • The hydroxyl group, -OH, is polar – (See module 4 for polarity) – Means it is hydrophilic or “water-loving” • Alcohols smaller than 4 carbons are soluble in water – Example is 2-carbon alcohol, ethyl alcohol found in alcoholic beverages. • Alcohols larger than 4 carbons are insoluble in water, such as cholesterol. 2. The Carbonyl Group The carbonyl is pronounced “car-bon-el” This is a double-bonded O attached to C ◦ =O At the end of a molecule called aldehyde ◦ CH3C=O called acetaldehyde H In middle of molecule, called ketone ◦ CH3-C-CH3 called acetone (fingernail polish O remover) The Carbonyl, continued • The double-bonded oxygen without an H means that it is less reduced (more oxidized) • The carbonyl is more oxidized than the hydroxyl but less than the carboxylic acid. • Carbonyls CANNOT form hydrogen bonds with itself because there is no H attached to the O. Examples of Aldehydes • Formaldehyde, a preservative • Acetaldehyde, a liver product • Glucose, a combination of aldehyde & alcohol Examples of Ketones • Carvone (caroway seeds) (also used as fungicide) • Thymine, a part of DNA --has 2 ketones 3. The Carboxyl Group The carboxyl group is a carbon to which a carbonyl AND a hydroxyl are added or -COOH or –CO2H The most oxidized of all the functional groups. Can form hydrogen bonds. Molecules with a carboxyl group are called carboxylic acids. Carboxylic Acids The H from the carboxyl is easily donated so carboxylic acids are acidic. CH3COOH CH3COO- + H+ acetic acid acetate hydrogen ion (proton) Carboxylic acids are the among the most acidic of all the organic, or carbon-containing, acids. Found frequently in living organisms. Examples of Carboxylic Acids • All amino acids, the subunits of proteins alanine • Some molecules of aerobic respiration, because the carboxyl group can come off as the waste gas CO2 which we exhale. Pyruvic acid, end of glycolysis Citric Acid, in Krebs cycle has 3 carboxyls 4. The Amino Group A molecule containing a Nitrogen often as – NH2 May be located at the end or in the middle of a molecule. Called amines. The Amines Can accept a proton (H+) and so are basic or alkaline. CH3NH2 + H+ CH3NH3+ methyl amine proton methylammonium ion Can also form hydrogen bonds with other polar groups. ◦ The hydrogen bonds holding DNA in the double helix involve amines. Are often the decay products of proteins and so smell terrible. Examples of Amines • All amino acids have an amino group: alanine • Decay molecules such as putrescine • Nucleotides such as thymine 5. Sulfhydryl Group Name is combination of “Sulfur” and “hydryl” or “hydrogen-containing”. Have –S-H attached to the middle or end of a molecule. Since sulfur is in the same family as oxygen, it forms 2 covalent bonds. Called thiols. An Example of Thiols The most important thiol in living organisms is the amino acid cysteine (cys) The thiols of 2 cysteines can make a covalent bond, the disulfide bridge, locking proteins into their characteristic shape. cys—CH2—S—S—CH2—cys 6. The Phosphate Group The phosphate group is a phosphorus atom with 4 oxygens attached. Two oxygens often donate their H’s, so phosphate can carry a 2- charge, an anion, a negatively-charged ion. Examples of Phosphates The Nucleic Acids all contain phosphates. – ATP, the energy molecule, has 3 phosphates, one of which may be used to transfer chemical energy between molecules. (See module on energetics) – DNA and RNA have backbones made of phosphates alternating with a deoxyribose or ribose monosaccharide. – Molecules that accept Hydrogen such as NAD+ or FAD+ contain phosphates. Some important functional groups of organic compounds FUNCTIONAL GROUP HYDROXYL CARBONYL CARBOXYL O OH (may be written HO C C OH ) STRUCTURE In a hydroxyl group (—OH), a hydrogen atom is bonded to an oxygen atom, which in turn is bonded to the carbon skeleton of the organic molecule. (Do not confuse this functional group with the hydroxide ion, OH–.) Figure 4.10 O The carbonyl group ( CO) consists of a carbon atom joined to an oxygen atom by a double bond. When an oxygen atom is doublebonded to a carbon atom that is also bonded to a hydroxyl group, the entire assembly of atoms is called a carboxyl group (—COOH). 20 Some important functional groups of organic compounds NAME OF COMPOUNDS Alcohols (their specific names usually end in -ol) Ketones if the carbonyl group is within a carbon skeleton Carboxylic acids, or organic acids Aldehydes if the carbonyl group is at the end of the carbon skeleton EXAMPLE H H H C C H H H OH H C H C H present in alcoholic beverages H C H Ethanol, the alcohol H O H Acetone, the simplest ketone H Figure 4.10 H H C C H H C H O C OH Acetic acid, which gives vinegar its sour tatste O C H Propanal, an aldehyde 21 • Some important functional groups of organic compounds AMINO SULFHYDRYL H N H Figure 4.10 O SH (may be written HS The amino group (—NH2) consists of a nitrogen atom bonded to two hydrogen atoms and to the carbon skeleton. PHOSPHATE ) O P OH OH The sulfhydryl group consists of a sulfur atom bonded to an atom of hydrogen; resembles a hydroxyl group in shape. In a phosphate group, a phosphorus atom is bonded to four oxygen atoms; one oxygen is bonded to the carbon skeleton; two oxygens carry negative charges; abbreviated P . The phosphate group (—OPO32–) is an ionized form of a phosphoric acid group (—OPO3H2; note the two hydrogens). 22 7. Methyl Group 8. Methylene Group 9. Ester group The ester functional group does not look much different next to the carboxylic acid functional group. In fact you might notice the only difference is the hydrogen atom, present in the carboxylic acid absent in the ester. This IS the key difference. Ester have carbon atoms in place of that hydrogen. • Esters have very pleasant odors. Banana Pineapple Concept Check 1. Identify the 3 functional groups in this molecule. 2. Which of the groups above form hydrogen bonds? 3. Which of the groups above is basic? Concept Check Answers 1. amino carboxyl hydroxyl 2. All three functional groups will form hydrogen bonds. 3. The amino group will accept a proton and so is basic.