Profiling Antidepressants
advertisement

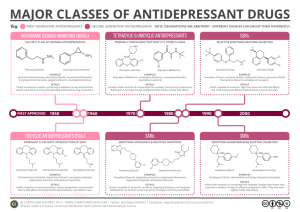
Profiling Antidepressants by Charles Vannoy What are antidepressants??? Drugs that are used to relieve or prevent psychic depression. Work by altering the way in which specific chemicals, called neurotransmitters, work in our brains (i.e. in the case of depression, some of the neurotransmitter systems don’t seem to be working properly). They increase the activity of these chemicals in our brains History Antidepressants were first developed in the 1950s and have been used on a regular basis since then. There are many different types, for example, the older tricyclics and the newer SSRIs (Selective Serotonin Reuptake Inhibitors). • These two types account for about 95% if the antidepressants prescribed. Now there are newer, more popular types, such as SNRI (Dual Serotonin and Norepinephrine Reuptake Inhibitor) and NDRI (Norepinephrine and Dopamine Reuptake Inhibitor) Antidepressants Available in the Market (Worldwide)1 1) Tricyclics and Tetracyclics (TCA) Imipramine Doxepin Desipramine Amoxepine Trimipramine Maprotiline Clomipramine Amitriptyline Nortriptyline Protriptyline 2) Monoamine Oxidase Inhibitors (MAOIs) Tranylcypramine Phenelzine Moclobemide 3) Serotonin Selective Reuptake Inhibitors (SSRIs) Fluoxetine Fluvoxamine Sertraline Paroxetine Citalopram 4) Dual Serotonin and Norepinephrine Reuptake Inhibitor (SNRI) Venlafaxine Duloxetine 5) Serotonin-2 Antogonist and Reuptake Inhibitors (SARIs) Nefazodone Trazodone 6) Norepinephrine and Dopamine Reuptake Inhibitor (NDRI) Bupropion 7) Noradrenergic and Specific Serotonergic Antidepressant (NaSSAs) Mirtazapine 8) Noradrenalin Specific Reuptake Inhibitor (NRI) Reboxetine 9) Serotonin Reuptake Enhancer Tianeptine Tricyclic Antidepressants (TCAs) Between 1960 and 1980 tricyclic antidepressants (TCAs) represented the major pharmacological treatment for depression. They have been considered a homogeneous group of drugs differing mostly in their potency to inhibit presynaptic norepinephrine or serotonin uptake and in their propensity for causing variety of unwanted effects. The TCAs induce anticholinergic, antihistaminergic, and cardiotoxic side effects which are related to their action on muscarinic (mainly M1), histamine (H1), adrenergic (α1) receptors and cardiac Na+ and Ca2+ channels. TCA Drugs The first tricyclic antidepressant discovered was imipramine, which was discovered accidentally in a search for a new antipsychotic in the late 1950s. Imipramine hydrochloride is a member of the dibenzazepine group of compounds. It is designated 5-[3-( Dimethylamino)propyl]10,11- ihydro-5H-dibenz [b,1-azepine] Monohydrochloride. Imipramine (Tofranil) Imipramine (Tofranil) Imipramine is the prototypic tricyclic antidepressant utilized in the treatment of major depression and exerts its therapeutic efficacy only after prolonged administration. The mechanism of action of imipramine hydrochloride is not definitely known. However, it does not act primarily by stimulation of the central nervous system. The clinical effect is hypothesized as being due to potentiation of adrenergic synapses by blocking uptake of norepinephrine at nerve endings. Amitriptyline (Elavil, Tryptanol, Endep) Amitriptyline HCl is 3-(10,11-dihydro-5Hdibenzo [a,d] cycloheptene-5ylidene)-N,Ndimethyl-1propanamine hydrochloride. Its empirical formula is C20H23N·HCl. Amitriptyline Amitriptyline cont’d Amitriptyline HCl is an antidepressant with sedative effects. Its mechanism of action is not known. It is not a monoamine oxidase inhibitor and it does not act primarily by stimulation of the central nervous system. Amitriptyline inhibits the membrane pump mechanism responsible for uptake of norepinephrine and serotonin in adrenergic and serotonergic neurons. Pharmacologically this action may potentiate or prolong neuronal activity since reuptake of these biogenic amines is important physiologically in terminating transmitting activity. This interference with the reuptake of norepinephrine and/or serotonin is believed by some to underlie the antidepressant activity of amitriptyline. Side Effects of TCAs The side effects of tricyclic antidepressant may include drowsiness, anxiety, restlessness, dry mouth, constipation, urinary retention or difficulty with urination, cognitive and memory difficulties, weight gain, sweating, dizziness, decrease in sexual ability and desire, muscle twitches, weakness, nausea, increased heart rate and irregular heart rhythms (rare). Some of these side effects relate to their anticholinergic properties. Selective Serotonin Reuptake Inhibitors (SSRIs) The SSRIs are currently the most widely utilized class of antidepressants in clinical practice. They act within the brain to increase the amount of the neurotransmitter, serotonin (5-hydroxytryptamine or 5-HT), in the synaptic gap by inhibiting its re-uptake. Instead of being discovered by accident, SSRIs were specifically designed while considering the biological causes of depression. SSRIs are described as 'selective' because they affect only the reuptake pumps responsible for serotonin, as opposed to earlier antidepressants, which affect other monoamine neurotransmitters as well. Because of this, SSRIs lack some of the side effects of the more general drugs. SSRI Drugs Fluoxetine Sertraline SSRI drugs include many of the popular drugs on the market today They include Fluoxetine (Prozac) and Sertraline (Zoloft). Fluoxetine (Prozac) Fluoxetine, also known as Prozac, was initially approved for treatment of depression in Belgium in 198617, and then Eli Lilly's Prozac was approved by the FDA on December 27th 1987 and introduced in the United States at the beginning of 1988. Prozac was the first of a new class of drugs, called selective serotonin reuptake inhibitors (SSRIs), to be approved for use in the United States. Fluoxetine hydrochloride is an antidepressant for oral administration. It is chemically unrelated to tricyclic, tetracyclic, or other available antidepressant agents. It is designated (±)-N-methyl-3-phenyl-3-[(a,a,a-trifluoro-ptolyl)-oxy]propylamine hydrochloride and has the empirical formula of C17H18F3NO•HCl. Fluoxetine (Prozac) cont’d Fluoxetine is a racemic mixture (50/50) of R-fluoxetine and Sfluoxetine enantiomers, where both enantiomers are specific and potent serotonin uptake inhibitors with essentially equivalent pharmacologic activity. But, the S-fluoxetine enantiomer is eliminated more slowly and is the predominant enantiomer present in plasma at steady state. The body eliminates Fluoxetine very slowly. The half-life of fluoxetine after a single dose is 2 days and after multiple dosing 4 days. The liver then metabolizes fluoxetine into norfluoxetine, a desmethyl metabolite, which is also a serotonin reuptake inhibitor; norfluoxetine has an even longer half-life, i.e. 8.6 and 9.3 days for single and repeated dosage respectively. Because fluoxetine's metabolism involves the P450IID6 system, concomitant therapy with drugs also metabolized by this enzyme system (such as the tricyclic antidepressants) may lead to drug interactions. Hence, the complexity of the metabolism of fluoxetine has several consequences that may potentially affect fluoxetine's clinical use. Fluoxetine Dosage and Side Effects It is marketed in capsules containing 10, 20, or 40 mg of active ingredient or in tablets containing 10 mg. Dosages in the range of 2060 mg per day are standard, with 80 mg considered a maximum. Fluoxetine has a wide range of published interactions, notably with monoamine oxidase inhibitors. Common side-effects include anxiety, restlessness, trembling, weakness, skin rash, anorgasmia, itching, and a decrease in sexual drive. Sertraline (Zoloft) Sertraline HCl is a selective serotonin reuptake inhibitor (SSRI) for oral administration. It is chemically unrelated to other SSRIs, tricyclic, tetracyclic, or other available antidepressant agents. Sertraline hydrochloride has the following chemical name: (1S-cis)-4-(3,4dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-1nanphthalenamine hydrochloride. The empirical formula is C17H17NCl2·HCl. Sertraline (Zoloft) cont’d The mechanism of action of sertraline is presumed to be linked to its inhibition of CNS neuronal uptake of serotonin (5HT). In vitro studies have shown that sertraline has no significant affinity for adrenergic (alpha1, alpha2, beta), cholinergic, GABA, dopaminergic, histaminergic, serotonergic (5HT1A, 5HT1B, 5HT2), or benzodiazepine receptors; antagonism of such receptors has been hypothesized to be associated with various anticholinergic, sedative, and cardiovascular effects for other psychotropic drugs. Sertraline does not inhibit monoamine oxidase. Sertraline undergoes extensive first pass metabolism. The principal initial pathway of metabolism for sertraline is Ndemethylation. N-desmethylsertraline has a plasma terminal elimination half-life of 62 to 104 hours. Sertraline Dosage and Side Effects Sertraline is manufactured by Pfizer as small green 25 mg tablets, blue 50 mg tablets, or off-yellow 100 mg tablets. It is used in dosages of between 25 mg and a maximum of 200 mg per day. It has a number of adverse effects including insomnia, asthenia, gastrointestinal complaints, tremours, confusion, and dizziness; it can induce mania or hypomania in around 0.5% of patients. One property of sertraline is that it appears to be also a minor inhibitor of dopamine reuptake. Serotonin and Noradrenaline Reuptake Inhibitors (SNRIs) Serotonin norepinephrine reuptake inhibitors (SNRIs) are a class of antidepressant used in the treatment of clinical depression and other affective disorders. They act upon two neurotransmitters in the brain that are known to play an important part in mood, namely, serotonin and norepinephrine. This can be contrasted with the more widelyused selective serotonin reuptake inhibitors (SSRIs), which act only on serotonin. SNRIs were developed more recently than SSRIs, and there are relatively few of them. Their efficacy as well as their tolerability appears to be somewhat better than the SSRIs, owing to their compound effect. These new drugs, because of their specificity for the serotonin and norepinephrine reuptake proteins, lack most of the adverse side effects of tricyclic antidepressants and monoamine oxidase inhibitors. SNRI Drugs SNRIs are currently some of the newest antidepressant drugs available on the market, and due to this there are only a few selected drugs that have been approved by the FDA for use. Venlafaxine Venlafaxine (Effexor) Venlafaxine hydrochloride is a prescription antidepressant first introduced by Wyeth in 1993, and marketed under the tradename Effexor®. It is used primarily for the treatment of depression, generalized anxiety disorder, and social anxiety disorder in adults. The chemical structure of Venlafaxine is designated (R/S)-1-[2(dimethylamino)-1-(4 methoxyphenyl)ethyl] cyclohexanol hydrochloride or (±)-1-[a [α- (dimethylamino)methyl] pmethoxybenzyl] cyclohexanol hydrochloride and it has the empirical formula of C17H27NO2 · HCl. Venlafaxine is the first and most commonly used SNRI. Venlafaxine (Effexor) cont’d Venlafaxine has a single chiral centre and exists as a racemic mixture of R-(–)- and S-(+)enantiomers. The R-enantiomer exhibits dual presynaptic inhibition of serotonin and noradrenaline reuptake, while the Senantiomer is predominantly a serotonin reuptake inhibitor. In vitro, venlafaxine is approximately 3- to 5-fold more potent at inhibiting serotonin than noradrenaline reuptake. It has low affinity for muscarinic cholinergic, histamine H1 and adrenergic receptors. Venlafaxine (Effexor) cont’d The mechanism of the antidepressant action of venlafaxine in humans is believed to be associated with its potentiation of neurotransmitter activity in the CNS. Venlafaxine and its active metabolite, Odesmethylvenlafaxine (ODV), are potent inhibitors of neuronal serotonin and norepinephrine reuptake and weak inhibitors of dopamine reuptake. Venlafaxine and ODV have no significant affinity for muscarinic, histaminergic, or a-1 adrenergic receptors Venlafaxine is primarily metabolized by the cytochrome P450 (CYP) 2D6 isoenzyme, with the CYP3A3/4 system providing a secondary metabolic pathway. The drug only modestly inhibits CYP2D6, and its metabolite has no effect on this isozyme. Venlafaxine Dosage and Side Effects Prescribed dosages are typically in the range of 75mg225mg per day, but higher dosages are sometimes used for the treatment of severe or treatment-resistant depression. Because of its relatively short half-life of 4 hours, Effexor should be administered in divided dosages throughout the day. Side effects may include nausea, dizziness, sleepiness, abnormal ejaculation, sweating,dry mouth, gas or stomach pain, abnormal vision, nervousness, insomnia, loss of appetite, constipation, confusion/agitation, tremors, and drowsiness. Norepinephrine and Dopamine Reuptake Inhibitor (NDRI) These are a class of antidepressants that are not really categorized as a special group of antidepressants. The only antidepressant in this group is Bupropion, which is an antidepressant of the aminoketone class, chemically unrelated to tricyclics or SSRIs. It is similar in structure to the stimulant cathinone, and to phenethylamines in general. Bupropion (Wellbutrin, [SR], [XL]) Bupropion was first synthesized by Burroughs Research in 1966, and patented by BurroughsWellcome (later GlaxoWellcome) in 1974. It was approved by the FDA in 1985 and marketed under the name Wellbutrin as an antidepressant. Bupropion is designated as (±)-1-(3-chlorophenyl)-2[(1,1-dimethylethyl)amino]-1propanone hydrochloride. The empirical formula is C13H18ClNO·HCl. Bupropion Bupropion (Wellbutrin, [SR], [XL]) cont’d Bupropion is a selective catecholamine (norepinephrine and dopamine) reuptake inhibitor. It has only a small effect on serotonin reuptake. It does not inhibit MAO. The actual mechanism behind bupropion's action is not known, but it is thought to be due to the effects on dopaminergic and noradrenergic mechanisms. Bupropion is metabolised in the liver. It has at least three active metabolites; hydroxybupropion, threohydrobupropion and erythrohydrobupropion. These active metabolites are further metabolised to inactive metabolites and eliminated through excretion into the urine. The half-life of bupropion is 20 hours as is hydroxybupropion's. Threohydrobupropion's half-life is 37 hours and erythrohydrobupropion's 33 hours. Bupropion and its Metabolites Bupropion Dosage Wellbutrin pills are available in three forms: immediate release, sustained release (SR) and extended release (XL). Generic forms of immediate and sustained release are available. Name Dosage Color Wellbutrin 75 mg yellow-gold Wellbutrin 100 mg red Wellbutrin SR 100 mg blue Wellbutrin SR 150 mg purple Wellbutrin SR 200 mg pink Zyban SR 150 mg purple Wellbutrin XL 150 mg white Wellbutrin XL 300 mg white Bupropion Side Effects Common side effects include dry mouth, tremors, anxiety, loss of appetite, agitation, dizziness, headache, excessive sweating, increased risk of seizure, and insomnia. Bupropion causes less insomnia if it is taken just before going to bed. Sexual side effects normally accompanying SSRI's do not accompany bupropion. Interestingly, patients commonly report increased libido, perhaps evidence of its dopaminergic properties. Conclusion Over the past half century there have been many new advances in antidepressants. Continued progress in understanding the neurobiology of antidepressant drugs will lead to further identification of the phenomenon of how the drugs act and work and development of more effective and faster acting therapeutic agents. Each day looks brighter and brighter for the advancement of newer and better antidepressants. References (1) Ayflegül, Y., Saffet, A.G., Tamam, L. Mechanism of actions of antidepressants: beyond the receptors. Klinik Psikofarmakoloji Bulteni, (2002), 12(4), 194-200. (2)Wellbutrin. GlaxoSmithKline. 15 Nov 2004 <http://us.gsk.com/products/assets/us_wellbutrinXLpdf> (3) Bupropion. Wikipedia Online Encyclopedia. 14 Oct 2004 http://en.wikipedia.org/wiki/Bupropion (4) Bupropion. Clinical Pharmacology. 10 Nov 2004 http://www.rxlist.com/cgi/generic/buprop_cp.htm (5) Wellbutrin. 2004. Encyclopedia of Medicine. HealthSquare. 14 Oct 2004 http://www.healthsquare.com/newrx/WEL1488.HTM (6) Fluoxetine. Wikipedia Online Encyclopedia. 14 Oct 2004 <http://en.wikipedia.org/wiki/Fluoxetine> (7) Fluoxetine. Clinical Pharmacology. 11 Nov 2004 http://www.rxlist.com/cgi/generic/fluoxetine_cp.htm (8) Prozac. 2004. Encyclopedia of Medicine. HealthSquare. 14 Oct 2004 <http://www.healthsquare.com/newrx/PRO1362.HTM> (9) Venlafaxine. Wikipedia Online Encyclopedia. 9 Nov 2004 http://en.wikipedia.org/wiki/Venlafaxine (10) Venlafaxine. Clinical Pharmacology. 12 Nov 2004 <http://www.rxlist.com/cgi/generic/venlafax_cp.htm> (11) Wellington K, Perry CM. Venlafaxine Extended Release: A Review of its Use in the Management of Major Depression. CNS Drugs (2001), 15, 643-669