Lecture 15b Oxides Hydroxides Carbonates and Phosphates mod 4
advertisement

Lecture 15b
Systematic Description of Minerals
Oxides, Hydroxides, Carbonates, Phosphates
Oxides
Three main groups based on metal cation/oxygen ratios (impurities not shown)
Hematite Group (X2O3)
most phases hexagonal
Corundum X=Al+3
Hematite X=Fe+3
Ilmenite X= (Fe+2,Ti+4) solid solution)
Rutile Group (XO2)
x- +4 cation
most phases tetragonal
Rutile X=Ti
Pyrolusite X=Mn
Cassiterite X=Sn
Uraninite X=U
Spinel Group (XY2O4)
Spinel X=Mg, Y=Al
X- +2
; Y- +3 cation
most phases isometric
or orthorhombic
Magnetite X=Fe+2, Y=Fe+3
Chromite X=Fe+2, Y=Cr
Chrysoberyl X=Be, Y=Al
Ulvospinel X=Ti+4, Y=Fe+2
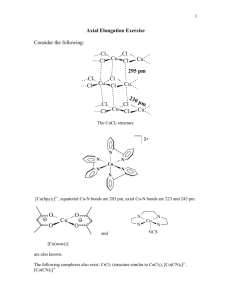
Hematite Group
X2O3
Hematite, ferric Irons Fe+3
in octahedral coordination
(C.N. = 6) with
Oxygen ions O-2
For example this one
Rutile Group
Rutile, Titanium ions Ti+4
in octahedral coordination
(C.N. = 6) with
Oxygen ions O-2
TiO2
Fe-Ti oxides
Anatase
Fe+2
Fe+3
Spinel Group
(XY2O4)
e.g Magnetite Fe+2Fe+32O4
Gem-quality
Spinels
Magnetite Fe3O4
Magnetite cubic unit cell = Fe24O32. Oxide ions CCP = FCC: 32
octahedral and 64 tetrahedral holes. Three types Iron ion present:
Fe3+ in tetrahedral holes (1/8 of the tetrahedral holes filled); Fe3+
in octahedral holes (1/4 of the octahedral holes filled); and Fe2+ in
octahedral holes (1/4 of the octahedral holes filled).
Other Common Oxides in color
Corundum (Al2O3)
Rutile (TiO2)
Hardness=9
Rutile needles in
Quartz
Pyrolusite (MnO2)
Blue = Sapphire
Red = Ruby
Chrysoberyl (BeAl2O4)
Mass of botryoidal
Pyrolusite
Pyrolusite dendrites
on surface
Cyclic twinning
in Chrysoberyl
Hydroxides
(OH)- main anionic group forming octahedrally coordinated
sheets with weak bonds between.
Two structural types:
Brucite-type Mg(OH)2
trioctahedral sheets (all
octahedral cation sites are
filled with Mg++)
Gibbsite-type Al(OH)3
dioctahedral sheets (only two
of three octahedral sites are
filled with Al+3) WHY?
Gibbsite Al(OH)3
Basic building block shown is Al(OH)3, shown as Al2(OH)6, electrical
neutrality is satisfied, so every third OH- octahedron must be empty
(no Al+3) Think of the missing OH- below as neutralizing the Al+3 below
Al+3 radius 0.61 A
OH- radius 1.37 A ratio 0.445 octahedral (CN = 6)
Common Types of Hydroxides
Brucite
Mg(OH)2
Gibbsite
Al(OH)3
Manganite
MnO(OH)
Diaspore
AlO(OH)
Goethite
FeO(OH)
Bauxite
Al-hydroxide*
Pronunciations:
Gear-tight
Go-eth-thite
Gurrr-tite
Seem to vary with
region
*mixture of diaspore, gibbsite, and boehmite (AlO(OH))
Carbonates
Reason for electrostatic valency calculations:
Amount of residual charge indicates relative strength of
bonds with cations, which are reflected in the hardness
of the mineral
Carbonates
Aragonite
(High-P) Orthorhombic
Calcite
(Low-P - Hexagonal)
Most are Hexagonal
Calcite Structure
Calcite
The structure of calcite is described as a "modified NaCl"
structure, but calcite is not cubic. The carbonate groups
stretch the atomic planes and distort the cube into a
rhombohedron.
Aragonite Group
Carbonate minerals with a single divalent cation of radius > 1.00 Å. With
increasing radius the species are Aragonite (CaCO3), Strontianite (SrCO3),
Cerussite (PbCO3), and Witherite (BaCO3). Aragonite is denser than calcite,
and is the high P polymorph. It crystallizes at ambient conditions and
persists metastably for millions of years. The orthorhombic structure is
nearly hexagonal with c as the unique axis.
Dolomite
CaMg(CO3)2
Forms during seasonal high tides that flood limestone (calcite) islands
with seawater. Mg++ in the seawater replaces some of the Calcium ions.
Has very distinctive crystals
Other Carbonates
Azurite - Cu3(CO3)2(OH)2 (Blue)
Malachite – Cu2CO3(OH)2 (Green)
Rhodochrosite – MnCO3
Borates
Kernite – Na2B4O6(OH)2·3H2O
H – 3; SG – 1.95
Borax - Na2B4O5(OH)4·8H2O
H – 2-2.5; SG – 1.7
Ulexite – NaCaB5O6(OH)6·5H2O
H – 1-2.5; SG – 1.96
“Television Rock”
Tungstates & Molybdates
Wolframite – (Fe,Mn)WO4
SG: 7-7.5
Scheelite – CaWO4
SG: ~6
Wulfenite – PbMoO4
SG: 6.8
Phosphates
Apatite – Ca5(PO4)3(F,Cl,OH)
prismatic hexagonal crystals
common in igneous rocks and
hydrothermal deposits
- variable colors “the deceiver”
Other Common Phosphates
Monazite – (Ce,La,Y,Th)PO4
Ore mineral for Rare Earth Elements
Useful mineral in U-Pb and Th age dating
Wavellite – Al3(PO4)2(OH)3·5H2O
Radiating globular aggregates
Turquoise –
CuAl6(PO4)4(OH)8·5H2O
A rare Chromate: Crocoite
PbCrO4
Monoclinic 2/m.
Commonly in prismatic crystals,
vertically striated
b=102o33’
Cleavage {110} perfect
H 2.5 – 3
G 5.9-6.1
Luster Adamantine
Color bright red to orange- red
Streak orange-yellow