Ch 5.1 The Nature of Chemical Reactions
advertisement

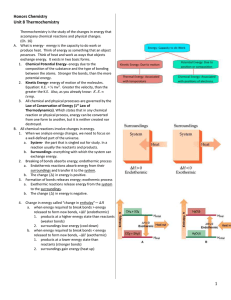
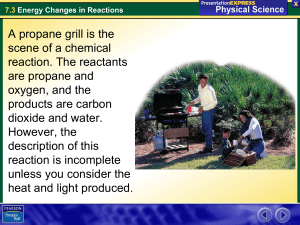
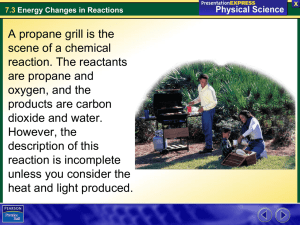
Ch 6.1 Chemical Reactions Objectives For this Chapter • Understand parts to a chemical equation (reactants, products, yeild sign, double arrow) • Conservation of matter is expressed through balancing chemical equations • Describe difference between endothermic and exothermic reactions Review from Ch 2 Chemical Change: Atoms in the reactants are rearranged to form one or more different substances • Old bonds are broken; new bonds form Ex) Fe and O2 form rust (Fe2O3) Ag and S form tarnish (Ag2S) Basic Chemical Equation -Objective ______ + _______ Reactants __________ Products Chemical Reaction A process in which at least one new substance is produced as a result of a chemical change Signs of a chemical reaction • In nature: grow, ripen, decay, burn • In the lab: bubbles, change in color, precipitate forms, light and heat produced • • • • • 1. The hydrogen peroxide oxidizes the phenyl oxalate ester, resulting in a chemical called phenol and an unstable peroxyacid ester. 2. The unstable peroxyacid ester decomposes, resulting in additional phenol and a cyclic peroxy compound. 3. The cyclic peroxy compound decomposes to carbon dioxide. 4. This decomposition releases energy to the dye. 5. The electrons in the dye atoms jump to a higher level, then fall back down, releasing energy in the form of light. Energy and Reactions • Energy needed to break bonds and form new compounds • Energy is released when new compounds are formed • Energy is conserved. Total energy before reaction = total energy of products Objective Exothermic reaction - reaction that gives off heat energy – feels hot Endothermic reaction - reaction that absorbs heat energy – feels cold