FP 1802 - Loyola College
advertisement
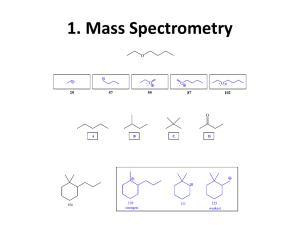
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 M.Sc. DEGREE EXAMINATION – FOOD CHEMISTRY FIRST SEMESTER – NOVEMBER 2010 FP 1802 - APPLICATIONS OF SPECTROSCOPY Date : 03-11-10 Time : 1:00 - 4:00 Dept. No. Max. : 100 Marks Part- A (10 2 = 20 marks) Answer ALL questions. 1. Define molar extinction coefficient. Give its unit. 2. Give the selection rule for IR activity 3. Calculate the number of modes of vibration for the following molecules (i) C6H6, (ii) CO2. 4. Which of the following molecules will absorb IR radiation? O2, I2, CO, HCl- why? 5. Define the term Chromophore. 6. How will you prove CO2 is linear by IR spectra? 7. How many types of orientations take place when a proton is placed in a magnetic field? 8. What is spin-spin splitting? 9. What is the principle involved in Mass spectroscopy? 10. What do you mean by base peak in a mass spectrum? Part – B Answer any EIGHT questions. (8 5 = 40 marks) 11. How will you determine the alkali metals by flame photometry? 12. Calculate the frequency of stretching vibration of C-C double bond, given that its force constant is 10 x 105 dynes cm-1. 13. Explain the principle of IR spectroscopy. 14. Derive Beer-Lambert’s law and explain the reasons for the deviation of the law. 15. Discuss the different types of interferences encountered in flame spectrometry. 16. Using Woodward-Fieser rules calculate λmax of the following molecules a) C CH CH CH3 b) 17. How is ferric ion estimated by using UV-visible spectrophotometer. 18. What is TMS? Explain its role in NMR spectra. 19. What is McLafferty rearrangement? Explain with mechanism. 1 20. Predict the fragmentation of Neopentane and draw the spectrum. 21. Discuss the Instrumentation of H1NMR. 22. A compound having molecular formula C9H11Br showed the following signals in H1NMR. Mutiplet (δ2.25)2H, Triplet (δ2.75)2H, Triplet(δ3.38)2H, Singlet (δ7.25) 5H Assign the structure. Part-C (4 10 = 40 marks) Answer any FOUR questions. 23. Draw and explain the working of flame photometer. 24. (a) Discuss the use of IR spectroscopy in the detection of hydrogen bonding in carboxylic acid. (b) The carbonyl stretching frequency of Benzamide is 1693 cm-1 whereas for methyl benzoate it is 1730 cm-1. Explain. 25. (a) How will you distinguish between cis and trans isomers of Cinnamic acid using IR spectroscopy? (b) Write a note on Fermi resonance. 26. What are the factors influencing Chemical shift? Explain them. 27. Give a brief description of Instrumentation of Mass spectroscopy. 28. Explain proton exchange reactions in H1NMR. **************** 2