Cardiac Output and Venous Return
advertisement

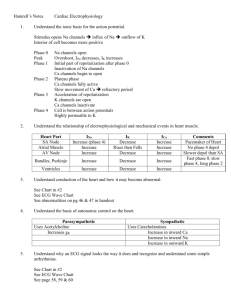
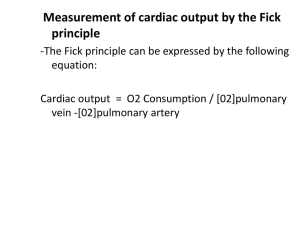
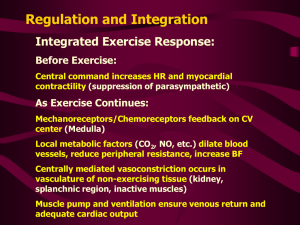
Cardiac Output and Venous Return Learning objectives • • • • • • • • Define Cardiac Output and Venous Return. Define the factors that affect cardiac output. Explain how alteration in (preload,contractility,afterload) change the cardiac output. Describe the effects of changing total peripheral resistance on cardiac output. Understand the principles underlying cardiac output measurements using the Fick principle, dye dilution, and thermodilution methods. Know how cardiac function (output) curves are generated and how factors which cause changes in contractility in the heart can alter the shape of cardiac function curves. Construct a vascular function curve. Predict how changes in total peripheral resistance, blood volume, and venous compliance influence this curve. Use the intersection point of the cardiac function curve and vascular function curve to predict how interventions such as hemorrhage, heart failure, autonomic stimulation, and exercise will affect cardiac output and right atrial pressure. Cardiac output • Amount of blood ejected by each ventricle per minute is called cardiac output (CO). Its value is almost same for both the ventricles & is about 5L/min. in a normal adult male • Cardiac output = heart beat rate X stroke volume (stroke volume is amount of blood ejected/ventricle/beat or stroke = EDV-ESV) • CO = 72/min X 70ml = 5 L/min (approx.) • Cardiac index: CI is the cardiac output per square meter of body surface area. Normal value is about 3 L/min/m2 Measurement of Cardiac Output • Calculation of flow through the pulmonary circuit provides a measure of the CO. Required data are: • oxygen consumption of the organ • A – V oxygen content (concentration) difference across the organ (not PO2) • In a test subject, oxygen consumption was measured at 700 mL/min.Pulmonary artery oxygen content was 140 mL per liter of blood and brachial artery oxygen content was 210 mL per liter of blood. Cardiac out-put was which of the following? a. 4.2 L/min b. 7.0 L/min c. 10.0 L/min d. 12.6 L/min e. 30.0 L/min Regulation of cardiac output • Factors effecting 14-14 Bainbridge Reflex, Atrial Receptors, and Atrial Natriuretic Peptide Respiratory Sinus Arrhythmia Determinants of Cardiac Output • Venous parameters, not arterial parameters, normally determine cardiac output. • Heart rate does not normally affect cardiac output but very low and very high heart rates impede venous return and cardiac output. • Increased resistance of arteries raises blood pressure but does not affect venous return and cardiac output. • For instance, aortic stenosis, coarc-tion of the aorta, and hypertension do not decrease cardiac output if the heart if able to pump against the increased afterload. Stroke Volume • Is determined by 3 variables: – End diastolic volume (EDV) = volume of blood in ventricles at end of diastole – Total peripheral resistance (TPR) = impedance to blood flow in arteries – Contractility = strength of ventricular contraction 14-9 Regulation of Stroke Volume • EDV is workload (preload) on heart prior to contraction – SV is directly proportional to preload & contractility • Strength of contraction varies directly with EDV • Total peripheral resistance = afterload which impedes ejection from ventricle – SV is inversely proportional to TPR Regulation of stroke volume 1. Preload :Passive tension in the muscle when it is being filled during diastole. • End diastolic volume • Venous return • Frank-Starling’s law (Energy of contraction is proportional to the initial length of cardiac muscle fibres) Venous Return • Is return of blood to heart via veins • Controls EDV & thus SV & CO • Dependent on: – Blood volume & venous pressure – Vasoconstriction caused by Symp – Skeletal muscle pumps – Pressure drop during inhalation Fig 14.7 14-15 Preload General features • Preload is the load on the muscle in the relaxed state. • More specifically, it is the load or prestretch on ventricular muscle at the end of diastole. • Preload on ventricular muscle is not measured directly; rather, indices are utilized. • Indices of left ventricular preload: – Left ventricular end-diastolic volume (LVEDV) – Left ventricular end-diastolic pressure (LVEDP) • somewhat less reliable indices of left ventricular preload are those measured in the venous system. – Left atrial pressure – Pulmonary venous pressure – Pulmonary wedge pressure • Measurement of systemic central venous pressure is an index of preload on the right ventricle Frank-Starling Law of the Heart (a) is state of myocardial sarcomeres just before filling Actins overlap, actinmyosin interactions are reduced & contraction would be weak In (b, c & d) there is increasing interaction of actin & myosin allowing more force to be developed • The preload effect can be explained on the basis of a change in sarcomere length Frank-Starling Law of the Heart • States that strength of ventricular contraction varies directly with EDV – Is an intrinsic property of myocardium – As EDV increases, myocardium is stretched more, causing greater contraction & SV 14-11 • The Frank–Starling law of the heart states that the stroke volume of the heart increases in response to an increase in the volume of blood filling the heart (the end diastolic volume) when all other factors remain constant. The contractility factor in systolic performance (inotropic state) • Contractility is the change in systolic performance at a given preload. • Contractility is a change in the force of contraction at any given sarcomere length. • Acute changes in contractility are due to changes in the intracellular dynamics of calcium. Indices of contractility –dp/dt (change in pressure vs. change in time) = rate of pressure development during isovolumetric contraction. –ejection fraction (stroke volume/end-diastolic volume) Effects of increased contractility • Both an increased preload and an increased contractility will be accompanied by an increased peak left ventricular pressure, but only with an increase in contractility will there be a decrease in the systolic interval. Regulation of stroke volume 3 Contractility (Inotropic state) Independence of preload and contractility Preload and Contraction in acute situation • In summary, A → B increased performance due entirely to preload A → C increased performance due entirely to contractility A → D increased performance due to an increase in both preload and contractility Regulation of stroke volume • 3 Afterload – • It is the load on the muscle during contraction • It represents the force that the muscle must generate to eject the blood into the aorta Indices of afterload • An approximation for the left ventricle is aortic diastolic pressure, which is primarily determined by the resistance of the arterioles (TPR). • Other acceptable indices of afterload on the left ventricle are the following: a. Mean aortic pressure b. Peak left ventricular pressure Regulation of stroke volume 2 Afterload • Usually measured as arterial pressure • ↑PR → ↑BP → ↑Afterload → ↓CO • ↓PR → ↓BP → ↓Afterload → ↑CO CHRONIC INCREASE IN AFTERLOAD Systolic dysfunction Diastolic dysfunction • An abnormal reduction in ventricular emptying due to impaired contractility or excessive afterload. • Decrease in ventricular compliance during the filling phase of the cardiac cycle due to either changes in tissue stiffness or impaired ventricular relaxation. • The consequence is a diminished FrankStarling mechanism An increase in afterload can be due to a pressure or a volume overload. Pressure Overload 1. Hypertension and aortic stenosis 2. Initially, increased performance due to (increased contractility); no decrease in CO 3. Chronically, in an attempt to normalize wall tension (actually internal wall stress), the ventricle develops a concentric hypertrophy. There is a dramatic increase in wall thickness and a decrease in chamber diameter. • The consequence of concentric hypertrophy (new sarcomes laid down in parallel, i.e., the myofibril thickens) • is a decrease in ventricular compliance and diastolic dysfunction, • followed eventually by a systolic dysfunction and ventricular failure. Volume Overload • mitral and aortic insufficiency, patent ductus arteriosus. • Chronically, in an attempt to normalize wall tension (actually internal wall stress), the ventricle develops an eccentric hypertrophy (new sarcomeres laid down end-to-end, i.e., the myofibril lengthens. All cardiac volumes increase.) • There is a modest increase in wall thickness that does not reduce chamber size. • Compliance of the ventricle is not compromised and diastolic function is maintained. • Eventual failure is usually a consequence of systolic dysfunction Effect of Various Conditions on Cardiac Output. No change • • Sleep Moderate changes in environmental temperature Increase • Anxiety and excitement (50– 100%) • Eating (30%) • Exercise (up to 700%) • High environmental temperature • Pregnancy • Epinephrine Decrease • Sitting or standing from lying position (20–30%) • Rapid arrhythmias • Heart disease The cardiac function (cardiac output) curve • A cardiac function curve is generated by keeping contractility constant and following ventricular performance as preload increases. • depicts the Frank-Starling relationship for the ventricle. • shows that cardiac output is a function of enddiastolic volume. The preload dependence of Cardiac output is defined by cardiac function curve • Starting at N, which represents a normal, resting individual: • A = decreased performance due to a reduction in preload • B = increased performance due to an increased preload • C represents an increased performance due almost entirely to increased contractility (close to the situation during exercise) • Points C, D, and E represent different levels of performance due to changes in preload only; all three points have the same contractility. Q • Haemorrhage and volume overload: how does it affects preload , performance and contractility? • Vector I: consequences of a loss in preload, e.g., hemorrhage, venodilators (nitro-glycerin) • Vector II: consequences of a loss in contractility, e.g., congestive heart failure • Vector III: consequences of an acute increase in contractility • Vector IV: consequences of an acute increase in preload, e.g., volume loading the individual going from the upright to the supine position Vascular function curves • Defines the changes in central venous pressure that are caused by changes in cardiac output. • The vascular function (venous return) curve depicts the relationship between blood flow through the vascular system (or venous return) and right atrial pressure. cardiac failure • What parameter is reduced in cardiac failure? cardiac contractility • How the kidneys are involved in the compensatory mechanism to cardiac failure. Sympathetic NS acts on Beta-1 cells in the kidney to release renin secretion. This increases blood volume and induces venoconstriction. • Q. How does cardiac failure affect CO and RAP? slight fall in CO Increase RAP Use the diagram below to answer the following three questions. The point marked control represents the state of the cardiovascular system in the resting state. An increase in total peripheral resistance and contractility is repre-sented by a shift from the resting state to point