Chem 20 Week One Schedule UPDATED Oct 10
advertisement
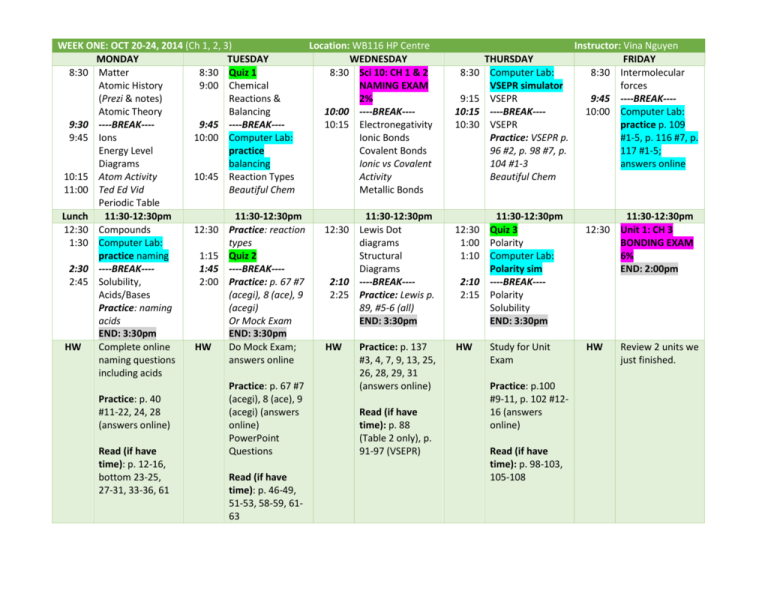
WEEK ONE: OCT 20-24, 2014 (Ch 1, 2, 3) Location: WB116 HP Centre MONDAY TUESDAY WEDNESDAY 8:30 Matter 8:30 Quiz 1 8:30 Sci 10: CH 1 & 2 Atomic History 9:00 Chemical NAMING EXAM (Prezi & notes) Reactions & 2% Atomic Theory Balancing 10:00 ----BREAK---9:30 ----BREAK---9:45 ----BREAK---10:15 Electronegativity 9:45 Ions 10:00 Computer Lab: Ionic Bonds Energy Level practice Covalent Bonds Diagrams balancing Ionic vs Covalent 10:15 Atom Activity 10:45 Reaction Types Activity 11:00 Ted Ed Vid Beautiful Chem Metallic Bonds Periodic Table Lunch 11:30-12:30pm 11:30-12:30pm 11:30-12:30pm 12:30 Compounds 12:30 Practice: reaction 12:30 Lewis Dot 1:30 Computer Lab: types diagrams practice naming 1:15 Quiz 2 Structural 2:30 ----BREAK---1:45 ----BREAK---Diagrams 2:45 Solubility, 2:00 Practice: p. 67 #7 2:10 ----BREAK---Acids/Bases (acegi), 8 (ace), 9 2:25 Practice: Lewis p. Practice: naming (acegi) 89, #5-6 (all) acids Or Mock Exam END: 3:30pm END: 3:30pm END: 3:30pm HW Complete online HW Do Mock Exam; HW Practice: p. 137 naming questions answers online #3, 4, 7, 9, 13, 25, including acids 26, 28, 29, 31 Practice: p. 67 #7 (answers online) Practice: p. 40 (acegi), 8 (ace), 9 #11-22, 24, 28 (acegi) (answers Read (if have (answers online) online) time): p. 88 PowerPoint (Table 2 only), p. Read (if have Questions 91-97 (VSEPR) time): p. 12-16, bottom 23-25, Read (if have 27-31, 33-36, 61 time): p. 46-49, 51-53, 58-59, 6163 8:30 9:15 10:15 10:30 12:30 1:00 1:10 2:10 2:15 HW Instructor: Vina Nguyen THURSDAY FRIDAY Computer Lab: 8:30 Intermolecular VSEPR simulator forces VSEPR 9:45 ----BREAK-------BREAK---10:00 Computer Lab: VSEPR practice p. 109 Practice: VSEPR p. #1-5, p. 116 #7, p. 96 #2, p. 98 #7, p. 117 #1-5; 104 #1-3 answers online Beautiful Chem 11:30-12:30pm Quiz 3 Polarity Computer Lab: Polarity sim ----BREAK---Polarity Solubility END: 3:30pm 11:30-12:30pm 12:30 Unit 1: CH 3 BONDING EXAM 6% END: 2:00pm Study for Unit Exam HW Practice: p.100 #9-11, p. 102 #1216 (answers online) Read (if have time): p. 98-103, 105-108 Review 2 units we just finished. Review – Naming formulas/compounds/reactions (Chapter 1 + 2) – 5% Classification of Matter Elements vs compounds vs molecules Atomic history and theory, ions, isotopes, energy level diagrams The Periodic Table of Elements Ionic vs molecular compounds and naming, polyatomic ions, hydrates, multivalent metals Solubility, acids and bases and naming Kinetic Molecular Theory, exothermic vs endothermic reactions Chemical reactions: formation, decomposition, combustion, simple replacement, double replacement Unit 1-Chemical Bonding in Matter (Chapter 3) – 15% Electronegativity, types of bonds Lewis diagrams, structural diagrams VSEPR, molecular polarity Solubility Intermolecular forces





